
Sumatriptan Sun
Ask a doctor about a prescription for Sumatriptan Sun

How to use Sumatriptan Sun
Leaflet accompanying the packaging: patient information
Sumatriptan SUN, 3 mg/0.5 ml, solution for injection in a pre-filled syringe
Sumatriptan
Before taking the medicine, carefully read the contents of the leaflet, as it contains important information for the patient.
- The leaflet should be kept, so that it can be re-read if necessary.
- In case of any doubts, the doctor or pharmacist should be consulted.
- This medicine has been prescribed for a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in the leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Sumatriptan SUN and what is it used for
- 2. Important information before taking Sumatriptan SUN
- 3. How to take Sumatriptan SUN
- 4. Possible side effects
- 5. How to store Sumatriptan SUN
- 6. Contents of the packaging and other information
1. What is Sumatriptan SUN and what is it used for
The active substance of Sumatriptan SUN is sumatriptan. It belongs to a group of medicines called 5HT receptor agonists.
This medicine is used for the acute treatment of migraine headache. Migraine symptoms may be caused by temporary swelling of blood vessels in the head. It is thought to work by constricting these blood vessels.
2. Important information before taking Sumatriptan SUN
When not to take Sumatriptan SUN
- if the patient is allergicto sumatriptan or any of the other ingredients of this medicine (listed in section 6)
- if the patient has heart problemsor has had a heart attack
- if the patient has problems with blood circulation in the arms and legs
- if the patient has had a stroke or mini-stroke(also known as a transient ischemic attack, TIA)
- if the patient has severe liver disease
- if the patient has moderate, severe, or mild uncontrolled high blood pressure
- with other medicines used to treat migrainecontaining ergotamine or similar medicines, such as methysergide, any triptan, or 5-HT agonist
- with MAOI (monoamine oxidase inhibitor) medicinesor if the patient has taken an MAOI in the last two weeks.
Warnings and precautions
Before starting treatment with Sumatriptan SUN, the patient should discuss with their doctor if:
- the patient has any of the following diseases: heart disease, including heart failure, angina pectoris, or coronary artery disease (heart attack), high blood pressure, liver or kidney disease, epilepsy, or brain disease. Especially women after menopause and men over 40 years old should have their heart and blood vessels checked before taking this medicine.
- the patient has risk factors for heart disease, such as heavy smoking or nicotine replacement therapy, especially in men over 40 years old and women after menopause. In very rare cases, severe heart disease has occurred after taking sumatriptan, even if the patients had no previous symptoms of heart disease. If any of these risk factors apply to the patient, it may indicate an increased risk of developing heart disease, so the patient's heart function should be checked before taking the medicine.
- the patient is allergic to certain antibiotics(sulfonamides); in people allergic to sulfonamides, an allergic reaction to sumatriptan may occur.
- the patient is taking medicines used to treat depression(so-called SSRI or SNRI) or lithium (a medicine used to treat manic-depressive disorders (bipolar)).
After discussing these issues, the doctor may still recommend taking the medicine, but will instruct the patient on how to use it in the form of an injection.
As with other migraine treatments, overuse of the medicine can worsen migraine symptoms and increase their frequency.
The medicine can only be used when the doctor has clearly diagnosed a migraine headache.
Immediate medical attention should be sought if symptoms such as confusion, rapid heartbeat, shivering, sweating, and muscle tremors occur. These may be symptoms of a very serious condition called "serotonin syndrome".
Sumatriptan SUN and other medicines
Before taking Sumatriptan SUN, the patient should inform their doctor if:
- the patient is taking migraine medicinescontaining ergotamine or its derivatives, such as ergotamine tartrate or methysergide maleate (if so, they should stop taking them at least 24 hours before taking sumatriptan)
- the patient is taking prescription depression medicines, such as MAOI or SSRI (including citalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline) or if the patient has taken an MAOI in the last 2 weeks
- the patient is taking lithium(a medicine used to treat manic-depressive disorders (bipolar))
- the patient is taking any prescription weight loss medicinesor epilepsy medicines
- the patient is taking any herbal productscontaining St. John's Wort (Hypericum perforatum). Taking this product with sumatriptan may increase the risk of side effects.
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Pregnancy and breastfeeding
The patient should consult their doctor or pharmacist before taking the medicine:
- if they are pregnant, think they may be pregnant, or plan to have a baby
- if they are breastfeeding. The doctor may still recommend taking sumatriptan, but the patient should avoid breastfeeding for 12 hours after taking the medicine and express and discard the milk during this time.
Driving and using machines
The medicine may cause drowsiness. If the patient experiences these symptoms, they should not drive or operate machinery.
Sumatriptan SUN contains sodium
The medicine contains less than 1 mmol of sodium (23 mg) per dose (3 mg), which means it is essentially "sodium-free".
3. How to take Sumatriptan SUN
The medicine should always be taken as directed by the doctor. In case of doubts, the doctor or pharmacist should be consulted.
Sumatriptan SUN is usually injected into the thigh or arm.
The patient should carefully read the section "How to use the pre-filled syringe" at the end of the leaflet.
The pre-filled syringe delivers the dose of medicine just under the skin quickly and painlessly. Injections MUST NOTbe performed in any other way than shown in the leaflet.
DO NOTinject Sumatriptan SUN into a vein.
Sumatriptan SUN MUST NOTbe used to prevent a migraine attack.
The pre-filled syringe should be used at the first symptoms of a migraine attack (although it will be just as effective if used at any time during the attack).
If migraine symptoms disappear and then return
If the symptoms of migraine disappear after the first dose of the medicine, but then return, a second pre-filled syringe can be used at any time within 24 hours, provided that at least one hour has passed since the first injection. No more than two pre-filled syringes should be used within 24 hours.
If migraine symptoms do not disappear
A second dose should not be taken during the same attack. However, the medicine can be taken during a subsequent attack at any time within the next 24 hours, provided that at least one hour has passed since the first injection. No more than two pre-filled syringes should be used within 24 hours.
If the injection does not relieve migraine symptoms, the patient can take ordinary painkillers, provided they do not contain ergotamine or its derivatives. After taking this medicine, the patient should wait at least six hours before taking any medicines containing ergotamine or its derivatives.
Use in children and adolescents (under 18 years old)
Sumatriptan should not be used in children and adolescents under 18 years old.
Use in elderly patients (over 65 years old)
There is limited experience with the use of sumatriptan in patients over 65 years old, so it is not usually prescribed in this age group.
Using a higher dose of Sumatriptan SUN than recommended
Taking a dose of the medicine higher than prescribed may lead to serious consequences.
In case of overdose, the patient should IMMEDIATELYcontact their doctor or the nearest emergency department.
In case of any doubts about taking this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
The following side effects have been reported (frequency not known).
If the following side effects occur, the patient shouldimmediately contact their doctor andnot take the medicine again, unless the doctor recommends otherwise
- sudden wheezing, trembling, or chest tightness, swelling of the eyelids, face, or lips, skin rash - red spots or hives (skin bumps), which may be symptoms of an allergic reaction
- seizure attack (usually in people with a history of epilepsy)
- inflammation of the large intestine (part of the intestine), which may manifest as pain in the lower left abdomen and/or bloody diarrhea with fever (ischemic colitis)
- Raynaud's phenomenon, which may manifest as pallor or blue discoloration of the skin and/or pain in the fingers of the hands, feet, ears, nose, or jaw in response to cold or stress
- chest pain (angina pectoris)
- heart attack
Other side effects
Very common (may affect more than 1 in 10 people)
- transient pain at the injection site
- prickling/stinging, redness, swelling, bruising, and bleeding at the injection site
Common (may affect less than 1 in 10 people)
- redness of the face (lasting a few minutes), dizziness, feeling of weakness, fatigue, drowsiness
- short-term increases in blood pressure shortly after taking the medicine
- nausea (feeling sick) or vomiting - when they are not part of a migraine attack
- pain, unusual sensations, including tingling, numbness, feeling of heat or cold, heaviness, pressure, or tightness. These symptoms usually resolve quickly, but may be intense and affect any part of the body, including the chest or throat. If these symptoms persist or are particularly bothersome, especially chest pain or pain in the heart area spreading to the arms, the patient should immediately consult their doctor, as in individual cases, these symptoms have been caused by a heart attack
- shortness of breath
- muscle pain
Very rare (may affect less than 1 in 10,000 people)
- liver function disorders: if the patient has had a blood test to check liver function and has taken sumatriptan, they should inform their doctor, as the medicine may affect the test results
Unknown (frequency cannot be estimated from the available data)
- tremors, muscle spasms, involuntary eye movements
- vision disturbances, including a feeling of flickering before the eyes, double vision, and visual impairment. There have also been reports of permanent vision damage.
- low blood pressure, which may lead to fainting, especially when standing up
- slow or rapid heartbeat, palpitations (feeling of rapid heartbeat), changes in heart rhythm
- diarrhea
- stiffness of the neck
- joint pain
- restlessness, excessive sweating
- In a patient who has recently had an injury or has an inflammatory condition (such as rheumatism or colitis), pain or worsening of pain at the site of injury or inflammation may occur.
- difficulty swallowing.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309
Website: https://smz.ezdrowie.gov.pl .
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Sumatriptan SUN
The medicine should be stored out of sight and reach of children.
The medicine should not be taken after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
There are no special storage instructions. The medicine should be stored in its original packaging to protect it from light.
The medicine should not be used if there are any visible particles in the solution.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Sumatriptan SUN contains
- The active substance of Sumatriptan SUN is sumatriptan. Each pre-filled syringe contains sumatriptan succinate equivalent to 3 mg of sumatriptan.
- The other ingredients are sodium chloride and water for injections.
What Sumatriptan SUN looks like and contents of the pack
The pre-filled syringe contains a clear, colorless or pale yellow solution for injection without visible particles. Each pack contains 1, 2, or 6 pre-filled syringes.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Netherlands
Manufacturer
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Netherlands
Terapia S.A.
Str. Fabricii nr 124
400632 Cluj-Napoca,
Romania
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany
Sumatriptan Lupin
Spain
Sumatriptán SUN
France
Sumatriptan SUN
Italy
Sumatriptan SUN Pharma
Netherlands
Sumatriptan SUN
Norway
Sumatriptan SUN
Romania
Sumatriptan SUN
Sweden
Sumatriptan SUN
United Kingdom
Sumatriptan
Date of last revision of the leaflet:08.09.2020
How to use Sumatriptan SUN, 3 mg/0.5 ml, solution for injection in a pre-filled syringe
The leaflet explains how to use the pre-filled syringe containing Sumatriptan SUN.
Before administering the injection, read the leaflet TWICE. If there are any questions, the patient should consult their doctor or pharmacist.
This applies only to patients who have been prescribed a dose of 3 mg.
View of the pre-filled syringe from the front
Figure 1
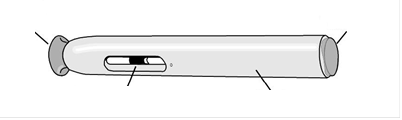
Figure 1
NOTES
- Checkthe appearance of Sumatriptan SUN in the control window. It should be a clear, colorless to pale yellow solution. Do not inject the solution if it is discolored, cloudy, or contains particles, flakes, or precipitates.
- Until the patient is ready to administer the medicine, do notremove the white needle shield from the pre-filled syringe.
- NEVERput the white needle shield back on the pre-filled syringe.
- NEVERpress the white needle shield with your thumb, fingers, or hand.
How to use the pre-filled syringe
a) Wash your hands thoroughly.
b) Find a comfortable, well-lit place and put everything you need within reach (pre-filled syringe, spirit or sterile swabs).
c) Find an injection site with sufficient subcutaneous tissue, such as the arm or thigh (Figure 2). Do not inject the medicine into areas where the skin is tender, bruised, red, or hard.
Figure 2
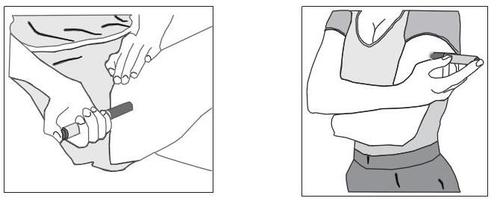
d) Wipe the injection site with spirit or a sterile swab and let the skin dry before administering the injection. Do not touch the injection site before administering the injection.
e) Remove the pre-filled syringe from its packaging.
f) Hold the pre-filled syringe with one hand and gently remove the white needle shield with the other hand (Figure 3). Do not twist the shield and do not put it back on, as this may damage the needle inside the pre-filled syringe.
Figure 3
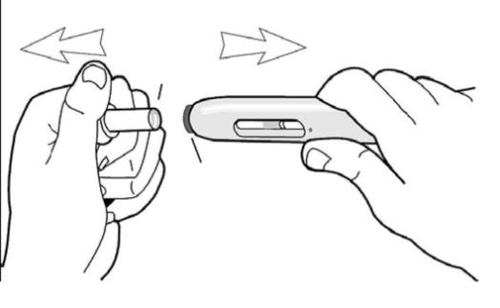
How to start the injection
- 1) Hold the open end of the pre-filled syringe against the injection site at a right angle (90º), press the safety shield firmly against the skin to unlock it. The pre-filled syringe will only work if the safety shield is completely hidden(Figure 4).
Hold the pre-filled syringe firmly against the skin.
Figure 4

- 2) Press and then release the blue activation button (first click). The injection of the medicine starts (Figure 5a).
Figure 5a and 5b
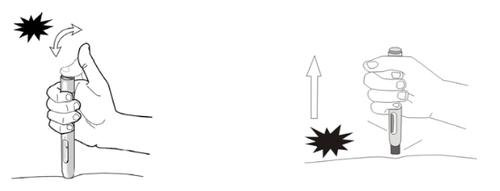
- 3) Do not remove the pre-filled syringe from the skin surface.
- 4) Wait until you hear the second click. The control window will turn blue, confirming that the injection is complete (Figure 5b).
- 5) Lift the pre-filled syringe straight up from the injection site. The injection is now complete.
If the control window is not blue, do not reuse the pre-filled syringe.
- 6) The safety shield on the pre-filled syringe will automatically extend to cover the needle and lock it in place. The needle will no longer be visible. There is no need to replace the white needle shield (Figure 6).
Figure 6
NEVER REUSE THE PRE-FILLED SYRINGE.
If there is a suspicion that the patient did not receive the full dose of the medicine, do not repeat the injection with a new pre-filled syringe.
- (7) If a drop of blood appears at the injection site, wipe it off with a swab or sanitary pad. Do not rub the injection site. If necessary, the injection site can be covered with a bandage.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterSun Pharmaceutical Industries Europe B.V. Terapia S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sumatriptan SunDosage form: Tablets, 50 mgActive substance: sumatriptanPrescription requiredDosage form: Tablets, 100 mgActive substance: sumatriptanPrescription requiredDosage form: Tablets, 100 mgActive substance: sumatriptanPrescription required
Alternatives to Sumatriptan Sun in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sumatriptan Sun in Spain
Alternative to Sumatriptan Sun in Ukraine
Online doctors for Sumatriptan Sun
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sumatriptan Sun – subject to medical assessment and local rules.














