
SUMATRIPTAN CFOURHEALTH 6 mg INJECTABLE SOLUTION IN PRE-FILLED PEN

How to use SUMATRIPTAN CFOURHEALTH 6 mg INJECTABLE SOLUTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Sumatriptan CFOURHEALTH 6 mg solution for injection in a pre-filled pen EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Sumatriptan CFOURHEALTH is and what it is used for
- What you need to know before you use Sumatriptan CFOURHEALTH
- How to use Sumatriptan CFOURHEALTH
- Possible side effects
- Storage of Sumatriptan CFOURHEALTH
- Contents of the pack and other information
- Instructions for use
1. What Sumatriptan CFOURHEALTH is and what it is used for
Sumatriptan CFOURHEALTH contains the active substance sumatriptan, which belongs to a group of medicines called triptans (5-HT1 receptor agonists).
Sumatriptan is used to treat migraine and a rare condition called cluster headache.
The cause of migraine and cluster headache symptoms may be the temporary widening of blood vessels in the head. Sumatriptan is thought to work by reversing this widening of blood vessels.
2. What you need to know before you use Sumatriptan CFOURHEALTH
Do not use Sumatriptan CFOURHEALTH
- if you are allergic to sumatriptan or any of the other ingredients of this medicine (listed in section 6).
- if you have heart problems, such as narrowing of the arteries (ischaemic heart disease) or chest pain (angina), or if you have had a heart attack.
- if you have circulation problems in your legs or arms, which cause cramp-like pains when you walk (peripheral vascular disease).
- if you have had a stroke or a mini-stroke (also called a transient ischaemic attack or TIA).
- if you have uncontrolled high blood pressure. You may be able to use Sumatriptan CFOURHEALTH if your high blood pressure is mild and being treated.
- if you have severe liver disease.
- with other medicines for migraine, including those containing ergotamine, or medicines similar to ergotamine (such as methysergide); or other triptans/5-HT1 receptor agonists (such as naratriptan, rizatriptan, or zolmitriptan; see section “Other medicines and Sumatriptan CFOURHEALTH”).
- with certain antidepressants (MAOIs) or if you have taken a MAOI in the last 2 weeks; see section “Other medicines and Sumatriptan CFOURHEALTH”.
If any of these apply to you:
Tell your doctor and do not use Sumatriptan CFOURHEALTH.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Sumatriptan CFOURHEALTH.
Additional risk factors
- If you are a heavy smoker or are using nicotine replacement therapy, and especially
- if you are a man over 40 years old, or
- if you are a woman who has passed the menopause
In very rare cases, people have developed serious heart conditions after using sumatriptan, even though they had no signs of heart disease before. If any of the above applies to you, it could mean that you have a greater risk of developing heart disease, so:
- Tell your doctor so that they can check your heart function before prescribing Sumatriptan CFOURHEALTH for you.
If you have a history of fits (seizures)
or if you have other conditions that may make it more likely that you will have a fit (such as head injury or alcoholism):
- Tell your doctor so that you can be closely monitored.
If you have high blood pressure
Sumatriptan may not be suitable for you.
- Tell your doctor or pharmacist before starting to use Sumatriptan CFOURHEALTH.
If you have liver or kidney disease
If any of these apply to you:
- Tell your doctor or pharmacist before starting to use Sumatriptan CFOURHEALTH.
If you are allergic to antibiotics called sulfonamides
If so, you may also be allergic to sumatriptan. If you know you are allergic to an antibiotic but are not sure if it is a sulfonamide:
- Tell your doctor or pharmacist before starting to use Sumatriptan CFOURHEALTH.
If you are taking antidepressants called SSRIs
(Selective serotonin reuptake inhibitors) or SNRIs (serotonin and noradrenaline reuptake inhibitors):
- Tell your doctor or pharmacist before starting to use sumatriptan. See also “Other medicines and Sumatriptan CFOURHEALTH” below.
The Sumatriptan CFOURHEALTH pen may contain latex
The transparent protective cap of the pen’s needle may contain latex.
- Tell your doctor if you are allergic to latex.
If you use Sumatriptan CFOURHEALTH frequently
Using Sumatriptan CFOURHEALTH too often can make your headaches worse.
- Tell your doctor if this applies to you. They may advise you to stop using sumatriptan.
If you feel pain or tightness in your chest after using Sumatriptan CFOURHEALTH
These effects may be intense but usually pass quickly. If they do not pass quickly, or get worse, seek medical help immediately. Section 4 (below) has more information on these possible side effects.
Children and adolescents
Sumatriptan CFOURHEALTH is not recommended for children and adolescents under 18 years. There is no experience.
Elderly (over 65 years)
Sumatriptan CFOURHEALTH is not recommended for people over 65 years.
Other medicines and Sumatriptan CFOURHEALTH
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Some medicines must not be taken with Sumatriptan CFOURHEALTH and others may cause adverse effects if taken with Sumatriptan CFOURHEALTH. You must tell your doctor if you are taking:
- ergotamine - also used to treat migraine, or medicines similar to ergotamine (such as methysergide) (see section 2 “Do not use Sumatriptan CFOURHEALTH”). Do not use Sumatriptan CFOURHEALTH at the same time as these medicines. Stop taking these medicines at least 24 hours before using Sumatriptan CFOURHEALTH. Do not take any medicine containing ergotamine or ergotamine-like compounds for at least 6 hours after using Sumatriptan CFOURHEALTH.
- other triptans/5-HT1 receptor agonists (such as naratriptan, rizatriptan, and zolmitriptan), which are also used to treat migraine (see section 2 “Do not use Sumatriptan CFOURHEALTH”). Do not use Sumatriptan CFOURHEALTH at the same time as these medicines. Stop taking these medicines at least 24 hours before using Sumatriptan CFOURHEALTH. Do not take another triptan/5-HT1 receptor agonist for at least 24 hours after using Sumatriptan CFOURHEALTH.
- MAOIs used to treat depression. Do not use Sumatriptan CFOURHEALTH if you have taken them in the last 2 weeks. See section 2 “Do not use Sumatriptan CFOURHEALTH”.
- SSRIs and SNRIs used to treat depression. Using Sumatriptan CFOURHEALTH with these medicines can cause serotonin syndrome (a set of symptoms including restlessness, confusion, sweating, hallucinations, increased reflexes, muscle spasms, shivering, increased heart rate, and trembling). Tell your doctor immediately if you are affected in this way.
- St John’s Wort (Hypericum perforatum). Taking herbal remedies containing St John’s Wort while using Sumatriptan CFOURHEALTH may increase the risk of side effects.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
There is only limited experience with the use of sumatriptan in pregnant women, although up to now there is no evidence of an increased risk of birth defects. Your doctor will decide if you can use Sumatriptan CFOURHEALTH during pregnancy.
Do not breast-feed your baby for 12 hours after using Sumatriptan CFOURHEALTH. If you express breast milk during this time, discard the milk and do not give it to your baby.
Driving and using machines
Both migraine symptoms and your medicine may make you feel drowsy. If you are affected, do not drive or operate machinery.
Sumatriptan CFOURHEALTH contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per 0.5 ml, i.e., essentially “sodium-free”.
3. How to use Sumatriptan CFOURHEALTH
Use Sumatriptan CFOURHEALTH exactly as your doctor has told you.
If you are not sure, ask your doctor or pharmacist.
Sumatriptan CFOURHEALTH is for subcutaneous use and is usually injected into the top of the arm or thigh.
There is a step-by-step guidefor using the pen at the end of this leaflet (see section 7 Instructions for use).
When to use Sumatriptan CFOURHEALTH
- It is best to use sumatriptan as soon as you feel a migraine or cluster headache coming on, although you can use it at any time during an attack.
- Do not use sumatriptan to try to prevent an attack, only use it after your migraine symptoms start.
How much to use
Adults aged 18 to 65 years
- The usual dose for adults aged 18 to 65 years with migraine or cluster headache is one 6 mg injection.
- If your symptoms start to come back, you can use a second Sumatriptan CFOURHEALTH injection if it has been at least 1 hour since the first injection.
- If the first injection does not work, do not use a second injection or any other sumatriptan injection for the same attack.
- If Sumatriptan CFOURHEALTH does not give you any relief, ask your doctor or pharmacist for advice.
If you use more Sumatriptan CFOURHEALTH than you should
Using too much sumatriptan could make you ill. If you have used more than two injections in 24 hours:
- Contact your doctor for advice.
In case of overdose or accidental ingestion, contact your doctor or pharmacist immediately or call the Toxicology Information Service, telephone: 91 562 04 20, stating the medicine and the amount taken.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Sumatriptan CFOURHEALTH can cause side effects, although not everybody gets them.
Some symptoms may be caused by the migraine itself.
Allergic reaction: seek medical help immediately
The following side effects have occurred, but their exact frequency is unknown.
- Signs of allergy include rash, itching, hives; wheezing; swelling of the eyelids, face or lips; collapse.
If you get any of these symptoms soon after using Sumatriptan CFOURHEALTH:
Do not use it again. Contact a doctor immediately.
Very common (may affect more than 1 in 10 people)
- Pain, swelling, or bruising at the injection site
Common (may affect up to 1 in 10 people)
- Pain, heaviness, pressure, or tightness in the chest, throat, or other parts of the body, or unusual sensations such as tingling, numbness, warmth, or cold. These effects may be intense but usually pass quickly.
If these effects continue or get worse (especially chest pain):
Seek medical help urgently. In a very small number of people, the cause of these symptoms may be a heart attack.
Other common side effects (may affect up to 1 in 10 people)
- Feeling sick (nausea) or being sick (vomiting), although this may be due to the migraine itself
- Feeling tired or sleepy
- Dizziness, feeling weak, or flushing
- Temporary increase in blood pressure
- Difficulty breathing
- Muscle pain
Very rare (may affect up to 1 in 10,000 people)
- Changes in liver function. If you have a blood test to check your liver function, tell your doctor or nurse that you are using sumatriptan.
Frequency not known (cannot be estimated from the available data)
- Seizures/fits, tremors, muscle spasms, stiffness of the neck
- Visual disturbances, such as flickering, reduced vision, double vision, loss of vision, and in some cases even permanent defects (although these may be due to the migraine itself)
- Heart problems, in which the heart beats more quickly, more slowly, or with an irregular rhythm, chest pain (angina), or heart attack
- Pale blue-tinged skin and/or pain in the fingers of the hands, toes, ears, nose, or jaw in response to cold or stress (Raynaud’s phenomenon)
- Feeling faint (blood pressure may fall)
- Pain in the lower left side of the stomach and bloody diarrhea (ischemic colitis)
- Diarrhea
- Pain in the joints
- Feeling anxious
- Excessive sweating
- If you have had a recent injury or if you have inflammation (such as arthritis or inflammation of the colon), you may experience pain or worsening of pain at the site of injury or inflammation.
- Difficulty swallowing
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Sumatriptan CFOURHEALTH
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and pen.
Do not store above 25°C. Store in the original package to protect from light.
Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to dispose of medicines no longer required.
These measures will help protect the environment.
6. Package Contents and Additional Information
Composition of Sumatriptan CFOURHEALTH
- The active ingredient is sumatriptan. Each pre-filled pen with 0.5 ml of solution contains 6 mg of sumatriptan (as sumatriptan succinate).
- The other ingredients are sodium chloride and water for injectable preparations.
Appearance of the Product and Package Contents
Sumatriptan CFOURHEALTH is a clear, colorless to pale yellow solution.
The pre-filled pen consists of a 1 ml long transparent glass syringe with a cut tip and a fixed needle (29 gauge, 12.7 mm, and 5 bevels) closed with a rigid transparent needle protector cap, a sterile rubber stopper in black chlorobutyl within a cardboard box.
Each box contains 1, 2, 6, or 12 pre-filled pens.
Only some pack sizes may be marketed.
Marketing Authorization Holder
C4 health GmbH
Wildstraße 20
89522 Heidenheim
Germany
Manufacturer
Hormosan Pharma GmbH
Hanauer Landstraße 139-143
60314 Frankfurt am Main
Germany
Venipharm
4, bureaux de la Colline
92210 Saint-Cloud
France
Abcur AB
Bergaliden 11,
Helsinborg, 252 23,
Sweden
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany | Sumatriptan C4 health 6 mg Injektionslösung im Fertigpen |
Denmark | Sumatriptan C4 health |
Finland | Sumatriptan C4 HEALTH 6mg injektioneste, liuos, esitäytetty kynä |
Italy | Sumatriptan C4 Health |
Netherlands | Sumatriptan C-Four 6 mg/0.5ml oplossing voor injectie in een voorgevulde pen |
Norway | Sumatriptan CFOURHEALTH |
Spain | Sumatriptán CFOURHEALTH 6mg/0.5ml solución inyectable en pluma precargada EFG |
Sweden | Sumatriptan C4 Health |
Date of the last revision of this leaflet: October 2023.
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Instructions for use
HOW TO USE THE PRE-FILLED PEN | Figure 1 (front view of the pre-filled pen) |
Sumatriptan CFOURHEALTH | |
This section explains how to use the sumatriptan pre-filled pen. Read it TWICE before starting the first step. If you have any doubts, consult your doctor or pharmacist. Only for use in patients who have been prescribed a dose of 6 mg. | |
PRECAUTIONS: | |
| |
| |
| |
| |
How to use the pre-filled pen | |
| |
| |
Do not inject into areas where the skin is sensitive, bruised, red, or hard. | Figure 2 |
| |
| |
| Figure 3 |
How to start the injection: | |
| |
| |
Do not move the pre-filled pen from the injection site. Continue to hold it firmly against the skin until an audible "click" signals the end of the injection. Keep the pen pressed against the skin for another 5 seconds to ensure that the full dose has been administered (Figure 4). | Figure 4 |
| Figure 5 |
| |
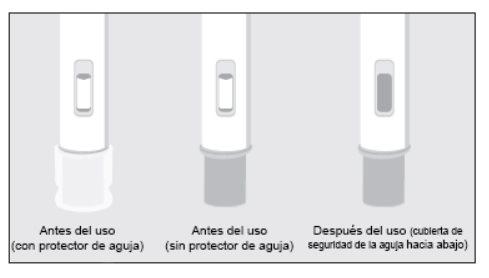
If the observation window is not gray, do not attempt to reuse the pre-filled pen. | |
NEVER ATTEMPT TO USE A PRE-FILLED PEN TWICE | |
| Figure 6 |
If you suspect that you have not received the full dose, do not repeat the injection using a new pre-filled pen. | |
|
Figure 1
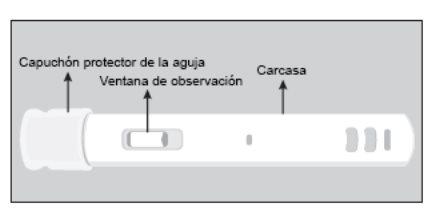
Figure 2
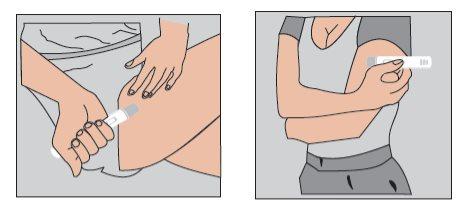
Figure 3
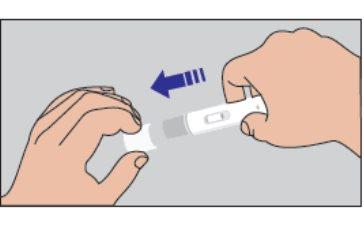
Figure 4
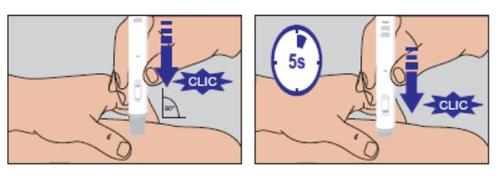
Figure 5
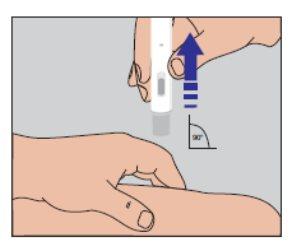
Figure 6
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SUMATRIPTAN CFOURHEALTH 6 mg INJECTABLE SOLUTION IN PRE-FILLED PENDosage form: NASAL PRODUCT, 10 mgActive substance: sumatriptanManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: NASAL PRODUCT, 20 mg sumatriptanActive substance: sumatriptanManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: INJECTABLE, 6 mgActive substance: sumatriptanManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for SUMATRIPTAN CFOURHEALTH 6 mg INJECTABLE SOLUTION IN PRE-FILLED PEN
Discuss questions about SUMATRIPTAN CFOURHEALTH 6 mg INJECTABLE SOLUTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















