
Sultiame Desitin
Ask a doctor about a prescription for Sultiame Desitin

How to use Sultiame Desitin
Leaflet accompanying the packaging: Patient information
Sultiame Desitin, 20 mg/ml, oral suspension
Sultiam
Before taking the medicine, carefully read the contents of the leaflet, as it contains important information for the patient.
- This leaflet should be kept. It may be necessary to read it again.
- In case of further questions, the doctor or pharmacist should be consulted.
- This medicine has been prescribed only for the patient. It should not be passed on to others. It may harm them, even if they have similar symptoms of the disease.
- If any side effects occur, the doctor or pharmacist should be contacted. This includes any possible side effects not listed in this leaflet. See section 4.
Table of contents of the leaflet
- 1. What is Sultiame Desitin and what is it used for
- 2. Important information before taking Sultiame Desitin
- 3. How to take Sultiame Desitin
- 4. Possible side effects
- 5. How to store Sultiame Desitin
- 6. Contents of the packaging and other information
1.
What is Sultiame Desitin and what is it used for
Sultiame Desitin contains the active substance sultiam, an antiepileptic drug used to treat a certain form of epilepsy.
Sultiame Desitin is used to treat self-limiting epilepsy with centrotemporal spikes (formerly known as Rolandic epilepsy) in children and adolescents from 3 years of age who do not respond to or tolerate other treatments or for whom other antiepileptic drugs cannot be used.
2. Important information before taking Sultiame Desitin
When not to take Sultiame Desitin
In case of hypersensitivity to sultiam, other sulfonamides, or any of the other ingredients of this medicine (listed in section 6).
In case of hyperthyroidism
In case of high blood pressure
In case of acute porphyria (a congenital or acquired disorder in which the body is unable to produce enough red blood cell pigment).
Warnings and precautions
Before starting treatment with Sultiame Desitin, it should be discussed with the doctor,
in case of kidney dysfunction,
in case of mental disorders.
In case of allergic reactions with symptoms such as fever, sore throat, rash with swelling of lymph nodes and (or) flu-like symptoms during treatment with Sultiame Desitin, the doctor should be consulted immediately and a blood morphology test should be ordered. In case of acute allergic reactions, the doctor may consider it necessary to discontinue Sultiame Desitin.
Before starting treatment with Sultiame Desitin, it is recommended to perform initial blood morphology, liver enzymes, and kidney function tests at weekly intervals in the first month of treatment, and then at monthly intervals. After six months of treatment, two to four control visits per year will be sufficient.
In a small number of patients treated with antiepileptic drugs, such as sultiam, thoughts of self-harm or suicide have occurred. If such thoughts occur, the doctor should be contacted immediately.
Sultiame Desitin and other medicines
The doctor or pharmacist should be informed about all medicines currently being taken by the patient or recently taken, as well as any medicines that the patient plans to take.
Sultiame Desitin and the following medicines or groups of medicines may interact with each other during combination therapy.
Combination of Sultiame Desitin with medicines used to treat epilepsy:
- -Phenytoin: Phenytoin levels in the blood may increase significantly. This combination requires close monitoring. Therefore, the doctor will frequently check the phenytoin levels in the blood, especially in case of kidney dysfunction.
- -Lamotrigine: In individual cases, lamotrigine levels in the blood may increase. Therefore, at the beginning of such combination therapy, lamotrigine levels in the blood should be monitored more frequently.
- -Primidone: The side effects of Sultiame Desitin may be exacerbated. In particular, it may cause unsteady gait, dizziness, and drowsiness.
- -Carbamazepine: There are indications of a decrease in sultiam levels in the blood when taken simultaneously with carbamazepine.
When taking sultiam simultaneously with other carbonic anhydrase inhibitors (e.g., topiramate used to treat epilepsy and migraines or acetazolamide used to treat increased intraocular pressure), due to carbonic anhydrase inhibition, the risk of side effects may increase.
Sultiame Desitin and alcohol
During treatment with Sultiame Desitin, alcohol should not be consumed, as it may unpredictably change and enhance the effect of Sultiame Desitin.
Sultiame Desitin, in interaction with alcohol, may also cause a very unpleasant reaction in some cases, characterized by vasodilation, pulsating headache, respiratory disorders, nausea, vomiting, rapid heartbeat, decreased blood pressure, vision disturbances, disorientation, shock, cardiac arrhythmias, loss of consciousness, and convulsions. These symptoms may be very diverse and of varying duration.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, suspects that she may be pregnant, or plans to have a child, she should consult a doctor or pharmacist before taking this medicine.
Pregnancy
There is an increased risk that this medicine may harm the unborn child. Therefore, it should not be taken during pregnancy unless it has been explicitly recommended by a doctor. Women of childbearing age taking Sultiame Desitin must use effective contraception.
Do not stop taking Sultiame Desitin without consulting a doctor. Sudden discontinuation of treatment or reduction of the dose without supervision may cause a recurrence of epileptic seizures, which may harm the pregnant woman and her unborn child.
Breastfeeding
It is not known whether the active substance contained in Sultiame Desitin passes into breast milk. For this reason, do nottake Sultiame Desitin while breastfeeding.
Driving and operating machinery
This medicine, even when taken as recommended, may affect the ability to react to a degree that impairs it, for example, the ability to drive vehicles or operate machinery.
This is especially true when combined with alcohol.
Sultiame Desitin contains sodium methyl parahydroxybenzoate (E219), sodium propyl parahydroxybenzoate (E217), sulfur dioxide (E220), sodium, fructose, glucose, and sucrose.
Sodium methyl parahydroxybenzoate (E219) and sodium propyl parahydroxybenzoate (E217) may cause allergic reactions (probably delayed).
Sulfur dioxide (E220) may rarely cause severe hypersensitivity reactions and bronchospasm.
This medicine contains 0.0026 mg of fructose per ml.
Glucose and sucrose: if the patient has previously been diagnosed with intolerance to some sugars, the patient should contact the doctor before taking the medicine.
Glucose, fructose, and sucrose may be harmful to teeth.
This medicine contains less than 1 mmol of sodium (23 mg) per ml, which means the product is considered "sodium-free".
3. How to take Sultiame Desitin
Treatment with Sultiame Desitin should be started and continued under the control of a doctor experienced in the treatment of epilepsy.
The medicine should always be taken as recommended by the doctor. In case of doubts, the doctor or pharmacist should be consulted.
Dose
The doctor usually starts with a small dose and gradually increases it over a week until an effective dose (called a maintenance dose) is reached. The usual maintenance dose is 5-10 mg (0.25-0.5 ml) per kilogram of body weight per day.
It is recommended to divide the daily dose into three single doses.
Table 1: Examples of dosing for initial dose of 2.5 mg sultiam per kg body weight per day
| Patient's weight | Initial dose: 2.5 mg* sultiam per kg body weight per day | |
| Single dose (given 3 times a day) | Total daily dose | |
| 0.5 – 0.75 ml (equivalent to 10 – 15 mg sultiam) | 1.5 – 2.25 ml (equivalent to 30 – 45 mg sultiam) |
| 0.75 – 1.0 ml (equivalent to 15 – 20 mg sultiam) | 2.25 – 3.0 ml (equivalent to 45 – 60 mg sultiam) |
| 1.0 – 1.25 ml (equivalent to 20 – 25 mg sultiam) | 3.0 – 3.75 ml (equivalent to 60 – 75 mg sultiam) |
| 1.25 – 1.5 ml | 3.75 – 4.5 ml |
| (equivalent to 25 – 30 mg sultiam) | (equivalent to 75 – 90 mg sultiam) | |
| 1.5 ml and more (equivalent to 30 mg sultiam and more) | 4.5 and above (equivalent to 90 mg sultiam and more) |
Table 2: Examples of dosing for maintenance dose of 5 mg sultiam per kg body weight per day
| Patient's weight | Maintenance dose: 5 mg* sultiam per kg body weight per day | |
| Single dose (given 3 times a day) | Total daily dose | |
| 1.0 – 1.5 ml (equivalent to 20 – 30 mg sultiam) | 3.0 – 4.5 ml (equivalent to 60 – 90 mg sultiam) |
| 1.5 – 2.0 ml (equivalent to 30 – 40 mg sultiam) | 4.5 – 6.0 ml (equivalent to 90 – 120 mg sultiam) |
| 2.0 – 2.5 ml (equivalent to 40 – 50 mg sultiam) | 6.0 – 7.5 ml (equivalent to 120 – 150 mg sultiam) |
| 2.5 – 3.0 ml (equivalent to 50 – 60 mg sultiam) | 7.5 – 9.0 ml (equivalent to 150 – 180 mg sultiam) |
| 3.0 ml and more (equivalent to 60 mg sultiam and more) | 9.0 and above (equivalent to 180 mg sultiam and more) |
Method and route of administration
Sultiame Desitin is intended for oral use.
Sultiame Desitin can be taken directly from the oral syringe or Sultiame Desitin can be taken immediately after mixing, preferably with a small amount of water or alternatively orange juice, milk, yogurt, or wheat porridge, or Sultiame Desitin can be administered through a tube.
Instructions for use
The instructions should be read carefully to know how to use this medicine.
Components of the dosing set
The dosing set consists of three parts:
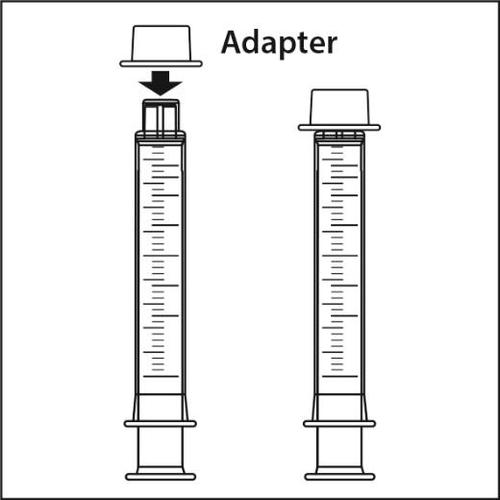
- 1.Plastic adapter
- 2.Oral syringe with a capacity of 10 ml, which fits into the plastic adapter.
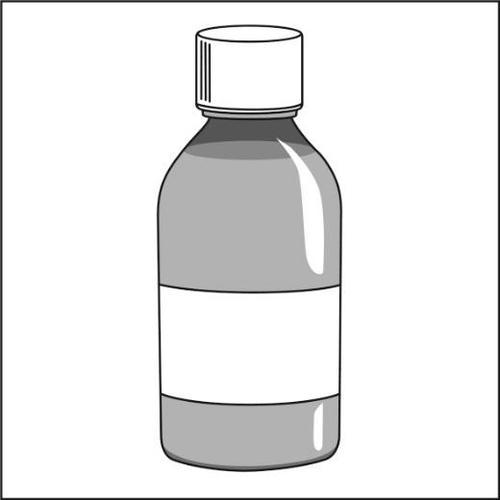
- 3.Bottle containing oral suspension with a child-resistant closure. After use, the cap should always be put back on.
Preparing the dose of the medicine
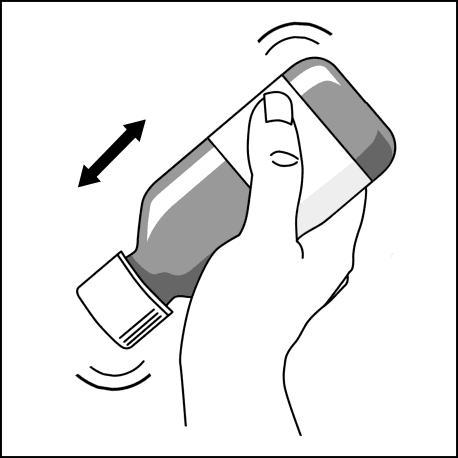
- 1. Vigorously shake the bottlefor 30 seconds in an inverted position. If sediment appears at the bottom of the bottle, it should be shaken for another 30 seconds.
- 2.Open the child-resistant closure by firmlypressing and twisting it in the opposite direction of the arrow (see the top of the cap).
Caution:The closure should be kept nearby to close the bottle after each use.
- 3.The bottle should be held vertically on the counter. The
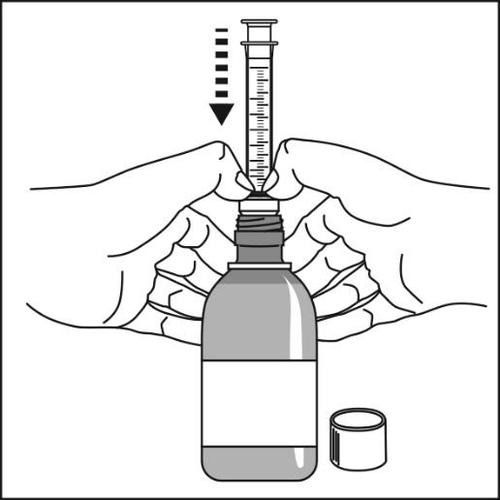
plastic adapter with the oral syringe should be firmlypressed into the bottle opening, as far as possible.
Caution:It may not be possible to fully press the adapter, but it will be pushed into the bottle after the cap is twisted.
After the first use, the adapter remains in the bottle.
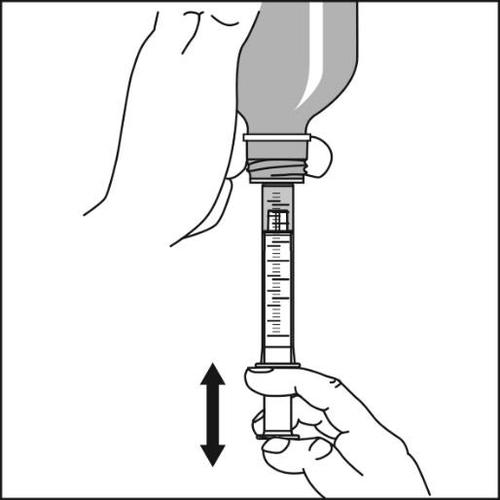
- 4.The oral syringe should be firmlyheld and the bottle should be carefully turned upside down. The plunger should be slowly pulled out so that the oral syringe fills with the suspension. Then, the plunger should be fully pressed back to remove any large air bubbles that may be inside the oral syringe.
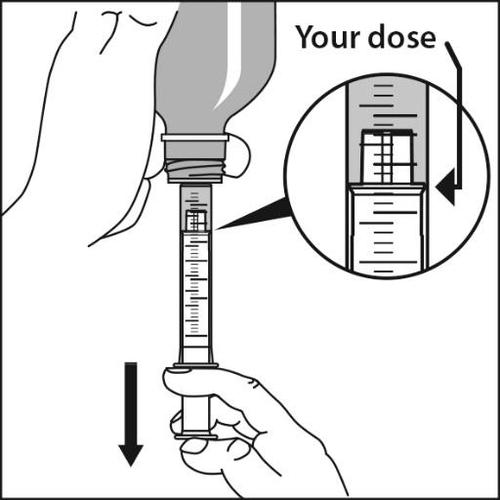
- 5.Withdraw the prescribed dose: The plunger should be slowly pulled out until the upper, wider part of the plunger is exactly at the mark on the oral syringe cylinder, which indicates the prescribed dose.
In case of doubts, the pharmacist should be consulted.
The dose can be taken directly from the oral syringe or Sultiame Desitin can be taken immediately after mixing, preferably with a small amount of water or alternatively orange juice, milk, yogurt, or wheat porridge. The mixture should be taken immediately.
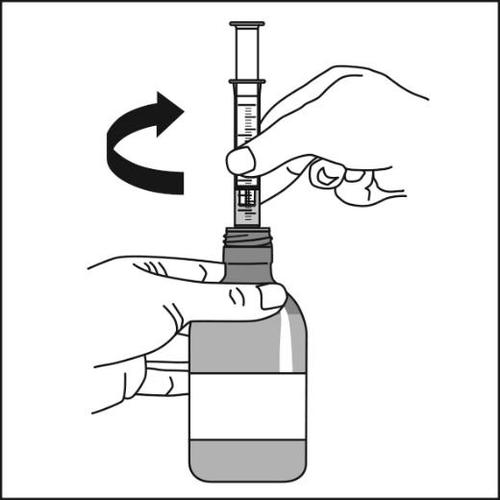
- 6.Carefully turn the bottle and oral syringe upside down and remove the oral syringe by gently twisting it out of the adapter.
The adapter must always remain in the bottle.
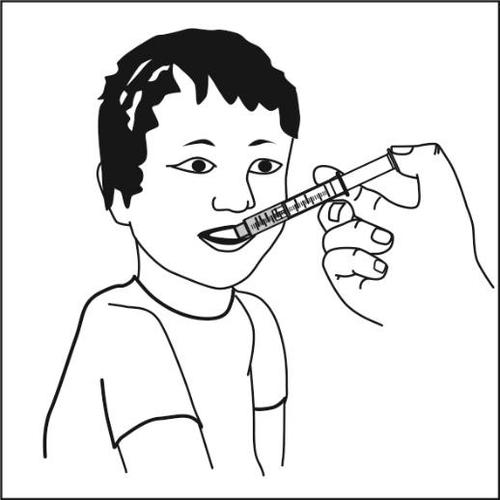
The dose should be given directly into the patient's mouth, who should be sitting upright. The plunger should be slowlypressed to facilitate swallowing. Immediately after administration, the patient should drink a glass of water, juice, or milk.
The dose can also be mixed with a smallamount of water or alternatively orange juice, milk, yogurt, or wheat porridge just before administration. The mixture should not be taken with carbonated drinks or hot foods to avoid belching or slowed swallowing. The entire mixture should be taken immediately.
Sultiame Desitin can be taken with food, but also without food. If possible, the routine of taking Sultiame Desitin should be maintained.
The oral suspension can also be administered through a tube, which should be rinsed with at least 15 ml of water immediately after administration. If this method of administration is used, the dose should be prepared in the manner described above immediately before administration.
How long should Sultiame Desitin be taken?
Antiepileptic treatment is essentially long-term therapy. In each individual case, the pediatric neurologist (neuropediatrician) experienced in the treatment of epilepsy should decide how to adjust the treatment, how long it should last, and when it should be discontinued. Sultiame Desitin should not be suddenly discontinued.
Taking a higher dose of Sultiame Desitin than recommended
The side effects listed in the "Possible side effects" section may be exacerbated.
In case of overdose, a doctor/ emergency medical doctor should be consulted as soon as possible and, if possible, show them the medicine and this leaflet.
Missing a dose of Sultiame Desitin
A double dose should not be taken to make up for a missed dose. The dose should be taken at the next scheduled time, as recommended by the doctor. The doctor should be informed about this.
Discontinuing Sultiame Desitin
If it is intended to discontinue or stop treatment with Sultiame Desitin, this should be discussed with the doctor first. The treatment with this medicine should not be discontinued without consulting a doctor, as this may jeopardize the effectiveness of the treatment and cause a recurrence of epileptic seizures.
The duration of treatment varies from person to person and will be determined by the doctor.
In case of any further questions about the use of this medicine, the doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common side effects (may affect more than 1 in 10 people):
- stomach problems (e.g., nausea, vomiting)
Common side effects (may affect up to 1 in 10 people):
breathing difficulties, and even respiratory failure (dose-dependent)
chest tightness, rapid heartbeat
tingling in hands, feet, or face (dose-dependent)
dizziness, headache
double vision
hiccups, weight loss, or loss of appetite
Uncommon side effects (may affect up to 1 in 100 people):
hallucinations, restlessness, apathy
muscle weakness, joint pain
exacerbated seizures, grand mal seizures
Unknown (frequency cannot be estimated from the available data):
delayed hypersensitivity reaction involving multiple organ systems with fever, skin rash, vasculitis, lymphadenopathy, joint pain, abnormal white blood cell count, as well as liver or spleen enlargement and severe skin reactions (Stevens-Johnson syndrome, toxic epidermal necrolysis)
acute kidney failure
worsening of vision, which may be significant, polyneuritis (inflammation of multiple nerves)
toxic reactions to the liver and (or) elevated liver enzymes
depressive mood/depression, personality changes, abnormal behavior (e.g., aggression, irritability, mood swings), and impaired cognitive abilities
diarrhea
In one patient with long-standing refractory epilepsy, taking Sultiame Desitin led to increasing limb weakness, increased salivation, slurred speech, and increasing drowsiness up to coma. The symptoms resolved within a few hours after discontinuation of Sultiame Desitin.
Sultiam belongs to a group of active substances (carbonic anhydrase inhibitors) that may lead to the formation of kidney stones, changes in blood composition (metabolic acidosis, hemodilution, and changes in serum electrolyte levels, such as decreased calcium levels in the blood), as well as fatigue/exhaustion.
Reporting side effects
If any side effects occur, the doctor or pharmacist should be informed, including any side effects not listed in the leaflet. See section 4.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw, phone: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Sultiame Desitin
This medicine should be stored out of sight and reach of children.
This medicine should not be used after the expiry date stated on the bottle and carton after EXP. The expiry date refers to the last day of the given month.
After the first opening of the bottle, it should not be used for more than 3 months.
This medicine should not be used if any damage to the bottle, closure, or carton is noticed.
Medicines should not be disposed of via wastewater or household waste. The pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Sultiame Desitin contains
The active substance is sultiam.
The other ingredients are: sodium methyl parahydroxybenzoate (E 219), sodium propyl parahydroxybenzoate (E 217), sucralose (E955), sodium docuzate, xanthan gum (E415), sodium dihydrogen phosphate dihydrate (E339), dipotassium phosphate (E340), strawberry flavor (containing gum arabic E414), flavor modifier (containing fructose, glucose, sucrose, sulfur dioxide (E 220)), masking flavor (containing sucralose E955, maltodextrin (from potato starch)), phosphoric acid concentrated (to adjust pH), purified water.
What Sultiame Desitin looks like and contents of the pack
Sultiame Desitin oral suspension is a white suspension.
A glass bottle with a child-resistant closure contains 200 ml or 250 ml of oral suspension. It is packed in a carton box containing an oral syringe with a capacity of 10 ml with a graduation of 0.25 ml and an adapter.
Not all pack sizes may be marketed.
Marketing authorization holder
Desitin Arzneimittel GmbH
Weg Beim Jäger 214
22335 Hamburg
Germany
Phone: +49 40 59101-0
Manufacturer
Desitin Arzneimittel GmbH
Weg Beim Jäger 214
22335 Hamburg
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium
Ospolot 20 mg/ml Suspension zum Einnehmen
Czech Republic
Ospolot
Denmark
Ospolot
Germany
Ospolot 20 mg/ml Suspension zum Einnehmen
Estonia
Ospolot
Spain
Ospolot 20 mg/ml Suspensión oral
Finland
Ospolot 20 mg/ml Oraalisuspensio
Ireland
Ospolot 20 mg/ml Oral suspension
Italy
Ospolot
Luxembourg
Ospolot Suspension zum Einnehmen
Netherlands
Ospolot 20 mg/ml Suspensie voor oraal gebruik
Norway
Ospolot
Poland
Sultiame Desitin
Portugal
Ospolot 20 mg/ml Suspensão oral
Romania
Ospolot 20 mg/ml Suspensie orală
Sweden
Ospolot 20 mg/ml Oral suspension
Slovakia
Ospolot 20 mg/ml Perorálna suspenzia
Date of last revision of the leaflet:06.03.2025
----------------------------------------------------------------------------------------------------------
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterDesitin Arznemittel GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sultiame DesitinDosage form: Tablets, 10 mgActive substance: brivaracetamPrescription requiredDosage form: Tablets, 25 mgActive substance: brivaracetamPrescription requiredDosage form: Tablets, 50 mgActive substance: brivaracetamPrescription required
Alternatives to Sultiame Desitin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sultiame Desitin in Spain
Online doctors for Sultiame Desitin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sultiame Desitin – subject to medical assessment and local rules.







