
Sudafed Xilosprai Ha

Ask a doctor about a prescription for Sudafed Xilosprai Ha

How to use Sudafed Xilosprai Ha
Leaflet attached to the packaging: patient information
Sudafed XyloSpray HA, 1 mg/ml, nasal spray, solution
Xylometazoline hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor or pharmacist. See section 4.
- If there is no improvement after 7 days or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet:
- 1. What is Sudafed XyloSpray HA and what is it used for
- 2. Important information before using Sudafed XyloSpray HA
- 3. How to use Sudafed XyloSpray HA
- 4. Possible side effects
- 5. How to store Sudafed XyloSpray HA
- 6. Package contents and other information
1. What is Sudafed XyloSpray HA and what is it used for
Xylometazoline is a vasoconstrictor that reduces swelling of the nasal mucosa, making it easier to breathe and facilitating the flow of secretions. The onset of action occurs within 2 minutes of administration. Sudafed XyloSpray HA contains sodium hyaluronate as an excipient, which protects and moisturizes the nasal mucosa. Sudafed XyloSpray HA is intended for use in adults and children over 6 years of age.
Indications for use
Reducing swelling of the nasal mucosa in acute rhinitis, vasomotor rhinitis, and allergic rhinitis. Facilitating the flow of secretions in sinusitis and otitis media associated with a cold.
2. Important information before using Sudafed XyloSpray HA
When not to use Sudafed XyloSpray HA:
- if the patient is allergic to xylometazoline hydrochloride or any other component of the medicine (listed in section 6);
- do not use in children under 6 years of age;
- do not use in patients after pituitary surgery and other surgical procedures involving exposure of the dura mater.
Warnings and precautions
The medicine should only be used after careful consideration by a doctor of the benefit-to-risk ratio in patients:
- being treated with monoamine oxidase inhibitors (MAOIs) or other medicines that may cause increased blood pressure;
- with increased intraocular pressure, especially in patients with narrow-angle glaucoma;
- with severe cardiovascular diseases (e.g., coronary heart disease, hypertension, long QT syndrome);
- with pheochromocytoma;
- with metabolic disorders (e.g., hyperthyroidism, diabetes);
- with prostatic hyperplasia;
- with hypersensitivity to substances with similar effects to xylometazoline, manifested by insomnia, tremors, dizziness, cardiac arrhythmias, and increased blood pressure. The medicine is intended for short-term use. Using xylometazoline in higher doses or for a longer period than recommended may lead to reactive hyperemia of the nasal mucosa. This causes narrowing of the airways, leading to frequent and prolonged use of the medicine by the patient. As a result, it can lead to drug-induced rhinitis and atrophy of the nasal mucosa.
Patient should consult a doctor if symptoms do not improve or worsen, or if new symptoms appear.
Other medicines and Sudafed XyloSpray HA
Patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. When administered intranasally, the likelihood of interactions with other medicines seems to be low, but they cannot be ruled out.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Driving and using machines
No effects of Sudafed XyloSpray HA on the ability to drive and use machines have been observed when used as recommended.
3. How to use Sudafed XyloSpray HA
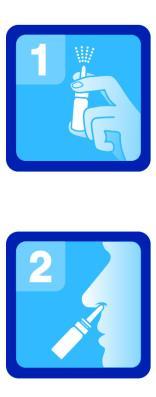
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist. In case of doubt, consult a doctor or pharmacist. Sudafed XyloSpray HA is intended for intranasal use only (also in otitis media). Adults and children over 6 years of age1 spray into each nostril up to 3 times a day. Do not administer more than 3 sprays into each nostril per day. Do not use in infants and children under 6 years of age. Do not use higher doses than recommended. Sudafed XyloSpray HA should not be used for more than 7 days, unless a doctor recommends otherwise. Repeated use of the medicine can be started after a few days' break. For the duration of use in children, consult a doctor. For hygiene reasons, one package of the medicine should be used by one patient only. Instructions for useRemove the plastic cap. Before first use, press the pump until a uniform mist of the sprayed medicine appears (fig. 1). This makes the spray ready for use. To administer a dose of the spray into the nose, place the tip of the applicator into the nostril, then press the pump once, while inhaling through the nose (fig. 2). During administration, hold the bottle in a position close to vertical. After use, replace the plastic cap.
Using a higher dose of Sudafed XyloSpray HA than recommended
In case of overdose or accidental oral administration, the following symptoms may occur: dilated pupils, nausea, vomiting, cyanosis (blue discoloration of the skin and mucous membranes), fever, seizures, tachycardia (accelerated heart rate), arrhythmias, circulatory collapse, cardiac arrest, hypertension, pulmonary edema (fluid accumulation in the lungs, manifested by shortness of breath), respiratory disorders, mental disorders. The following symptoms may also occur: central nervous system depression with drowsiness, decreased body temperature, bradycardia (slow heart rate), decreased blood pressure as in shock, apnea, and coma. The medicine should be kept out of the reach of children. In case of overdose, seek medical help immediately. The doctor will take appropriate actions, such as administering activated charcoal. In justified cases, gastric lavage and oxygen therapy may be necessary. To lower blood pressure, the doctor may recommend administering phentolamine in a dose of 5 mg in a 0.9% sodium chloride solution by slow intravenous infusion or 100 mg orally. The use of vasoconstrictor medicines is contraindicated. If necessary, antipyretic and anticonvulsant medicines should be used.
Missing a dose of Sudafed XyloSpray HA
Do not use a double dose to make up for a missed dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The frequency of side effects is presented according to the following scheme: Very common occurs in less than 1 in 10 patients Common occurs in less than 1 in 100 patients Uncommon occurs in less than 1 in 1,000 patients Very common side effects may include symptoms of irritation (burning or dryness of the nasal mucosa), sneezing, especially in sensitive patients. After discontinuation of the medicine, there may be an exacerbation of nasal congestion (reactive hyperemia). Common side effects may include: local nasal administration may cause systemic effects, such as palpitations, accelerated heart rate, increased blood pressure, nasal bleeding. Uncommon side effects may include nausea, vision disorders, allergic reactions, headache, insomnia, or fatigue. Prolonged or frequent use, as well as administration in higher doses than recommended, may lead to reactive hyperemia with drug-induced rhinitis, manifested by a feeling of nasal congestion, nasal discharge, nasal itching, and sneezing. This effect may occur after 5 days of treatment, and if the use of the medicine continues, it may lead to atrophic rhinitis. Reporting side effects If side effects occur, including any side effects not listed in the leaflet, the patient should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C 02-222 Warsaw Tel.: +48 22 49 21 301 Fax: +48 22 49 21 309 Website: https://smz.ezdrowie.gov.pl Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information on the safety of the medicine can be collected.
5. How to store Sudafed XyloSpray HA
Keep the medicine out of the reach and sight of children. Store in a temperature below 25°C. The shelf life after opening the bottle is 12 months. Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month. Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Sudafed XyloSpray HA contains
- The active substance is xylometazoline hydrochloride. 1 ml of Sudafed XyloSpray HA solution contains 1 mg of xylometazoline hydrochloride.
- The other ingredients are: sodium hyaluronate, sorbitol, glycerol, sodium dihydrogen phosphate dihydrate, disodium phosphate dihydrate, sodium chloride, water for injections.
What Sudafed XyloSpray HA looks like and contents of the pack
The packaging of Sudafed XyloSpray HA consists of a white HDPE bottle with a dosing pump (3K system), containing 10 ml of solution (at least 55 doses), in a cardboard box.
Marketing authorization holder and manufacturer
Marketing authorization holder:
McNeil Healthcare (Ireland) Limited Office 5, 6 & 7, Block 5 High Street, Tallaght Dublin 24, D24 YK8N Ireland
Manufacturer:
Famar Health Care Services Madrid, S.A.U. Avda. Leganés, 62 28923 Alcorcón-Madrid Spain Ursapharm Arzneimittel GmbH Industriestraße 35 66129 Saarbrücken Germany For more information, please contact: email: [email protected]
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterFamar Health Care Services Madrid SAU Ursapharm Arzneimittel GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sudafed Xilosprai HaDosage form: Aerosol, 1mg/mlActive substance: xylometazolineManufacturer: Jadran-Galenski laboratorij d.d.Prescription not requiredDosage form: Aerosol, 0.5 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not requiredDosage form: Aerosol, 1 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not required
Alternatives to Sudafed Xilosprai Ha in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sudafed Xilosprai Ha in Ukraine
Alternative to Sudafed Xilosprai Ha in Spain
Online doctors for Sudafed Xilosprai Ha
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sudafed Xilosprai Ha – subject to medical assessment and local rules.







