

Sudafed Xilosprai dla dzieci

Ask a doctor about a prescription for Sudafed Xilosprai dla dzieci

How to use Sudafed Xilosprai dla dzieci
Package Leaflet: Information for the Patient
Warning! Keep the Leaflet! Information on the Immediate Packaging in a Foreign Language.
Sudafed XyloSpray for Children (Olynth)
0.5 mg/ml, nasal spray, solution
Xylometazoline hydrochloride
Sudafed XyloSpray for children and Olynth are different trade names for the same medicine.
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor or pharmacist.
- You should keep this leaflet, so you can read it again if you need to.
- If you need advice or more information, you should ask your pharmacist.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. See section 4.
- If after 3 days there is no improvement or you feel worse, you should contact your doctor.
Table of Contents of the Leaflet
- 1. What is Sudafed XyloSpray for children and what is it used for
- 2. Important information before using Sudafed XyloSpray for children
- 3. How to use Sudafed XyloSpray for children
- 4. Possible side effects
- 5. How to store Sudafed XyloSpray for children
- 6. Contents of the pack and other information
1. What is Sudafed XyloSpray for children and what is it used for
Sudafed XyloSpray for children contains xylometazoline, which helps to constrict the blood vessels inside the nasal cavity, reducing the swelling of the nasal mucosa (nasal lining) and making it easier to breathe.
The spray is used for the short-term treatment of nasal congestion or sinusitis with a runny nose, occurring in the course of a cold or sinusitis.
2. Important information before using Sudafed XyloSpray for children
When NOT to use Sudafed XyloSpray for children:
DO NOT use the spray in children under 2 years of age.
Warnings and precautions
Before starting to use the medicine, you should discuss it with your doctor or pharmacist if:
- you have a strong reaction to sympathomimetics (substances similar to adrenaline), as the use of Sudafed XyloSpray for children may cause insomnia, dizziness, uncontrolled muscle tremors, irregular heartbeat, or increased blood pressure;
- you have heart disease (e.g., long QT syndrome), high blood pressure, or circulatory system disease;
- you have hyperthyroidism, diabetes, or other metabolic system disease;
- you have adrenal gland disease;
- you have prostate enlargement (prostatic hyperplasia).
Continuous useof the medicine for a long period may cause worsening of sinusitis or nasal mucosa inflammation.
Sudafed XyloSpray for children and other medicines
Before starting to use Sudafed XyloSpray for children, you should consult your doctor or pharmacist.
DO NOT take the medicine while using:
- certain antidepressants, such as tricyclic and tetracyclic antidepressants, monoamine oxidase inhibitors, and within two weeks of using monoamine oxidase inhibitors (see "When NOT to use Sudafed XyloSpray for children");
- other medicines that increase blood pressure.
You should tell your pharmacist about all medicines you are taking or have recently taken, including those that are available without a prescription.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, you should ask your doctor or pharmacist for advice before using this medicine.
DO NOT use Sudafed XyloSpray for children during pregnancy due to the lack of data on its effects on the fetus.
Breastfeeding women should consult their doctor before using the medicine.
It is not known whether the active substance of this medicine passes into breast milk.
Driving and using machines
No effects of Sudafed XyloSpray for children on the ability to drive and use machines have been observed.
Sudafed XyloSpray for children contains benzalkonium chloride
The medicine contains 0.2 mg of benzalkonium chloride per ml, which corresponds to 0.014 mg/0.070 ml (dose). Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time.
3. How to use Sudafed XyloSpray for children
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor or pharmacist. If you are unsure, you should ask your doctor or pharmacist.
Children from 2 to 12 years of age
Unless your doctor or pharmacist has advised otherwise, you should use one dose of Sudafed XyloSpray for children into each nostril, up to 3 times a day for no longer than 7 days. DO NOT exceed the recommended dose.
- 1. Remove the protective cap. Before the first use, press the pump several times until a uniform mist of the sprayed medicine appears (see Fig. 1). The medicine is now ready for use.
- 2. Hold the bottle in a position close to vertical. Insert the applicator into the nostril – do not spray the medicine if the tip of the applicator is below the nostril (see Fig. 2).
- 3. Press the pump once, while inhaling through the nose. Repeat the same steps when administering the medicine to the other nostril.
- 4. After use, replace the protective cap on the bottle.
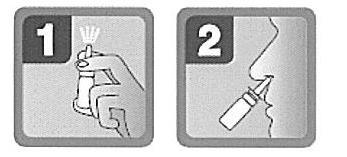
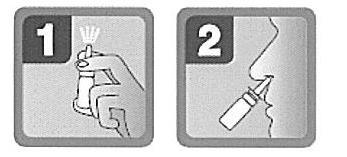
To minimize the risk of spreading infections, the packaging of the medicine should not be used by more than one person, and the applicator should be rinsed after each use.
DO NOT use the medicine in children under 2 years of age.
Using a higher dose of Sudafed XyloSpray for children than recommended
You should immediately contact your doctor, pharmacist, or the nearest emergency department, taking the packaging of the medicine or this leaflet with you.
Using a higher dose of the medicine than recommended may cause central nervous system depression, e.g., loss of muscle function, fatigue, dry mouth, sweating, rapid and irregular heartbeat, and increased blood pressure.
Missing a dose of Sudafed XyloSpray for children
If you miss a dose, you should take it as soon as possible. However, if it is almost time for the next dose, you should not take the missed dose, but continue using the medicine as directed. DO NOT take a double dose to make up for the missed dose.
If you have any further questions about using this medicine, you should ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
STOPusing the medicine and immediately consult your doctorif you experience any of the following side effects, which may be symptoms of an allergic reaction:
- difficulty breathing or swallowing, swelling of the face, lips, tongue, or throat;
- itching with red rash or blisters.
Other side effects:
Common (occurring in less than 1 in 10 people):
- feeling of stinging or burning in the nose and throat, and dryness of the nasal mucosa
Uncommon (occurring in less than 1 in 100 people):
- nasal bleeding
Rare (occurring in less than 1 in 1000 people):
- headache, increased blood pressure, nervousness, nausea, dizziness, insomnia, and palpitations
- transient vision disturbances and systemic allergic reactions
Frequency not known (frequency cannot be estimated from the available data):
- worsening of sinusitis or nasal mucosa inflammation after stopping the use of the medicine
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Sudafed XyloSpray for children
The medicine should be stored out of sight and reach of children.
Do not store above 30°C.
After the first opening of the packaging of Sudafed XyloSpray for children, do not store for more than 12 weeks.
DO NOT use Sudafed XyloSpray for children after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Sudafed XyloSpray for children contains
The active substanceof the medicine is xylometazoline hydrochloride. 1 ml of the solution contains 0.5 mg of xylometazoline hydrochloride. One dose (0.070 ml) of Sudafed XyloSpray for children contains 35 micrograms (or 0.035 mg) of xylometazoline hydrochloride.
The other ingredientsare: benzalkonium chloride, solution, sorbitol liquid, non-crystallizing, sodium chloride, sodium dihydrogen phosphate dihydrate, disodium phosphate dihydrate, disodium edetate, purified water.
What Sudafed XyloSpray for children looks like and contents of the pack
The spray is a clear, colorless solution in a 10 ml glass bottle with a dosing pump, in a cardboard box.
For more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Germany, the country of export:
Johnson & Johnson GmbH
Johnson & Johnson Platz 2
41470 Neuss
Germany
Manufacturer:
Johnson & Johnson GmbH
Johnson & Johnson Platz 2
41470 Neuss
Germany
Delpharm Orléans
5, avenue de Concyr
45071 Orléans Cedex 2
France
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
Pharma Innovations Sp. z o.o.
ul. Jagiellońska 76
03-301 Warsaw
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Marketing authorization number in Germany, the country of export:6460123.00.00
Parallel import authorization number:230/18
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Poland Sudafed XyloSpray for children
Romania Olynth 0.5 mg/ml nasal spray, solution
Date of leaflet approval: 16.05.2023
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Johnson&Johnson GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sudafed Xilosprai dla dzieciDosage form: Aerosol, 1mg/mlActive substance: xylometazolineManufacturer: Jadran-Galenski laboratorij d.d.Prescription not requiredDosage form: Aerosol, 0.5 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not requiredDosage form: Aerosol, 1 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not required
Alternatives to Sudafed Xilosprai dla dzieci in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sudafed Xilosprai dla dzieci in Ukraine
Alternative to Sudafed Xilosprai dla dzieci in Spain
Online doctors for Sudafed Xilosprai dla dzieci
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sudafed Xilosprai dla dzieci – subject to medical assessment and local rules.







