

Staloral

Ask a doctor about a prescription for Staloral

How to use Staloral
Leaflet attached to the packaging: patient information
STALORALPlant-based allergen extracts
STALORALAnimal-based allergen extracts
STALORALHouse dust mite allergen extracts
STALORALFungal allergen extracts (mold fungi, dermatophytes, yeast)
STALORALMixtures of allergen extracts (plant-based, animal-based, mite, fungal)
Oral and sublingual solution
10; 100 IR/ml or 10; 100 IC/ml
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their disease symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is STALORAL and what is it used for
- 2. Important information before using STALORAL
- 3. How to use STALORAL
- 4. Possible side effects
- 5. How to store STALORAL
- 6. Contents of the packaging and other information
1. What is STALORAL and what is it used for
STALORAL is a medicine for specific immunotherapy, which is used in patients allergic to plant-based allergens (plant pollen), animal-based allergens, house dust mites, or fungi. A complete list of allergens is attached in Annex 1 to this leaflet.
Indications for use
Allergic diseases of a seasonal or year-round nature, characterized by rhinitis (sneezing, runny nose, or itchy nose, stuffy nose), conjunctivitis (itchy and watery eyes), or asthma (mild or moderate) in adults and children (over 5 years old).
The goal of treatment with STALORAL is to increase immunological tolerance to allergens, and thus alleviate allergic reactions.
2. Important information before using STALORAL
When not to use STALORAL
Only a doctor can determine if there are contraindications to using the medicine.
Warnings and precautions
Before starting treatment, it is necessary to stabilize allergy symptoms with appropriate symptomatic treatment, if necessary.
Before starting treatment with STALORAL, you should inform your doctor or pharmacist:
STALORAL and other medicines
You should tell your doctor about all the medicines you have taken recently, including those that are available without a prescription.
Before starting treatment, you should inform your doctor:
Symptomatic treatment (e.g., with antihistamines and/or nasal corticosteroids) may be used concurrently with STALORAL.
Pregnancy and breastfeeding
Pregnant women, or women who suspect they are pregnant, or plan to become pregnant, should consult their doctor or pharmacist before using this medicine.
Breastfeeding women should consult their doctor or pharmacist before using this medicine.
There is no experience with the use of STALORAL in pregnant or breastfeeding women, so immunotherapy should not be initiated in pregnant or breastfeeding women unless the doctor considers it necessary.
Women who become pregnant or start breastfeeding during treatment with this medicine should consult their doctor about continuing to take this medicine.
Driving and using machines
There are no contraindications to driving or using machines while being treated with STALORAL.
STALORAL contains sodium chloride
In the case of patients on a low-sodium diet, the presence of sodium chloride in the medicine should be taken into account (one vial, i.e., 10 ml of solution, contains 590 mg of sodium chloride). See "Warnings and precautions".
3. How to use STALORAL
This medicine should always be used in accordance with the doctor's recommendations. In case of doubts, you should consult a doctor or pharmacist.
Specific immunotherapy is most effective when started in the early stages of the disease.
The dose size does not depend on age, but on the degree of individual patient reactivity.
In the case of seasonal allergies, it is recommended to start treatment before the pollen season and continue until the end of the pollen season.
In the case of year-round allergies, it is recommended to continue treatment throughout the year.
Treatment is divided into two stages:
- basic treatment - with gradual dose increase
- maintenance treatment - with a constant dose.
Before each administration of the medicine, you should carefully check the information on the packaging and ensure that the data matches the doctor's recommendation (prescription): patient's name, composition and concentration of the medicine, as well as check the expiry date of the medicine.
Basic treatment with dose increase
The medicine is used daily, with the dose increased every day until the maintenance dose is reached, according to the following scheme:
The above dosing scheme should be considered as a guideline and example of treatment. Treatment may be modified depending on the patient's condition and their reaction to treatment.
Maintenance treatment - constant dose
The maximum well-tolerated dose should be taken daily or 3 times a week.
Duration of treatment
Usually, desensitization treatment is used for 3 to 5 years.
The doctor will re-evaluate the treatment in case of no significant improvement in symptoms after a year (year-round allergy) or after the first pollen season (seasonal allergy).
Method of administration
To ensure safe use of the medicine, the doctor's instructions should be strictly followed.
STALORAL is administered sublingually.
The medicine should be taken during the day, on an empty stomach, without food and drink.
Before each use of the medicine, you should check the expiry date and ensure that the used vial matches the doctor's recommendation (prescription)
You should use the dosing pump mechanism to administer the appropriate amount of solution directly into the mouth under the tongue.
Wait 2 minutes before swallowing the solution.
The doctor may recommend a different dosing schedule depending on the patient's condition and their reaction to treatment.
STALORAL should be administered to children by adults.
Information about the first use of the medicine:
For safety reasons and to ensure that the vial remains intact, the vials are hermetically sealed with plastic and aluminum caps.
You should always check if the medicine matches the one prescribed by the doctor. In case of doubts, you should consult a doctor.
It is extremely important to read the instructions for using the dosing pump carefully. If necessary, you should ask the doctor for detailed explanations.
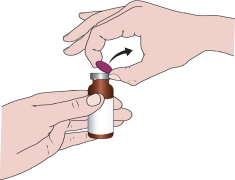
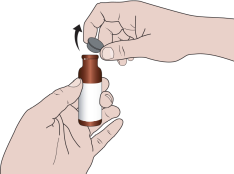
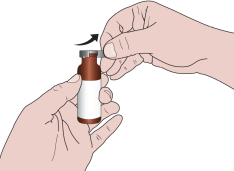
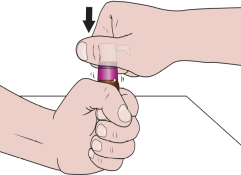
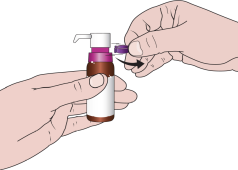
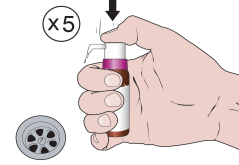
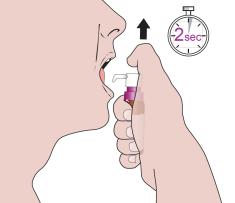
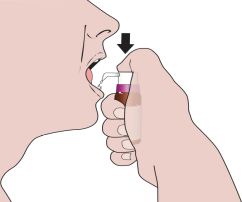
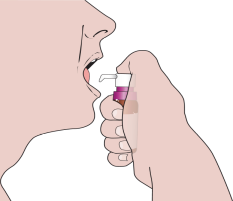
When using the medicine for the first time, you should follow the steps below:
- 1. Remove the plastic, colored part of the cap.
- 2. Pull the metal tab and completely remove the aluminum cap.
- 3. Remove the gray plug.
- 4. Remove the dosing pump from the plastic cover. Place the vial on a flat surface, and then, holding it firmly with one hand, press the pump firmly into place.
- 5. Remove the purple protective ring.
- 6.
Activate the pump by pressing the plunger 5 times.
The released solution should be discarded.
The dosing pump measures 1 full dose of the medicine only after 5 presses.
- 7. Place the tip of the dosing pump in the mouth, directly under the tongue.
- 8. Press the plunger with a smooth motion until it stops. Use the finger that can press the hardest.
- 9. Release the pressure on the plunger completely. You should ensure that there is at least a 2-second interval between consecutive presses.
- 10. You should repeat the above procedure until the number of presses prescribed by the doctor is reached. Hold the solution under the tongue for 2 minutes and swallow.
- 11. After use, clean the tip of the dosing pump and put on the protective ring.
During subsequent uses, you should remove the protective ring and repeat the steps starting from point 7.
Use in children
Allergen immunotherapy is not recommended for children under 5 years old.
Using a higher dose of STALORAL than recommended
You should immediately inform your doctor or pharmacist.
In case of using a higher dose than recommended, the risk of side effects increases, including systemic reactions or severe local reactions.
Missing a dose of STALORAL
It is recommended to take the medicine in the usual dose the next day.
You should not take a double dose to make up for the missed dose.
If you forget to take several consecutive doses, you should contact the doctor who prescribed the treatment to determine a new treatment schedule.
Discontinuing STALORAL
In case of discontinuing STALORAL for less than a week, you can continue treatment using the last dose. In case of discontinuing treatment for more than a week, you should contact your doctor.
In case of any further doubts related to the use of this medicine, you should consult a doctor or pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
During treatment with STALORAL, the patient will be exposed to the action of substances that can cause allergic reactions at the site of administration and/or symptoms that may affect the whole body.
Such reactions may occur at the beginning of treatment or later, during treatment.
You should immediately discontinue STALORAL and seek medical attention if you experience or notice the following symptoms:
Severe allergic reaction with rapid onset of symptoms, which affects the whole body, e.g., intense itching or rash, difficulty breathing, abdominal pain, or symptoms related to low blood pressure, such as dizziness, malaise.
Tolerance to a given dose may change over time, depending on the patient's health and environment.
In case of a side effect, you should consult a doctor, who may change the treatment schedule.
The doctor may prescribe pre-treatment with anti-allergic medicines to reduce the frequency and severity of side effects.
The following additional side effects have also been observed:
Frequent (affecting less than 1 in 10 patients)
Mouth disorders (e.g., swelling, discomfort, tingling, itching, numbness, blisters, ulcers), tongue swelling, throat discomfort or pain or swelling or irritation, nasal congestion (stuffy nose, runny nose, sneezing, itchy nose, discomfort in the nose), cough, salivary gland disorders, itchy eyes, itchy ears, nausea, vomiting, abdominal pain, diarrhea, skin redness or itching.
Uncommon (affecting less than 1 in 100 patients)
Eye disorders (redness, irritation, tearing), herpes, hoarseness, breathing difficulties, asthma, hives, atypical skin allergy symptoms (burning, tingling, pricking), gastritis, esophageal spasm.
Rare (affecting less than 1 in 1000 patients)
Headache, eczema, joint pain, muscle pain, weakness, enlarged lymph nodes, fever.
Additionally, the following side effects have been reported:
Taste disorders, dry mouth, dizziness, facial swelling, severe allergic reactions, esophagitis.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, you should inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: 22 49 21 301, fax: 22 49 21 309, e-mail: [email protected].
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store STALORAL
Before opening: Store in a refrigerator (2°C - 8°C).
After first opening: Store in a refrigerator (2°C - 8°C) for up to 30 days.
During transport, the vials should always be carried in a vertical position.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after the "EXP" date and more than 30 days after first opening. The expiry date refers to the last day of the given month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What STALORAL contains
The active substance of the medicine is allergen extracts or their mixtures in combination with mannitol. The amount of mannitol does not exceed 40 mg/ml.
1 vial (10 ml) of solution contains allergen extracts (according to Annex 1) at a concentration of:
10; 100 IR*/ml (standardized allergen extract)
or
10; 100 IC**/ml (non-standardized allergen extract)
The qualitative composition of the active substance can be individually selected for the patient within the range of allergens listed in Annex 1.
*/**) The activity of the relevant allergen expressed in IR/ml or IC/ml is given on the label.
- Other ingredients are: sodium chloride, glycerol, purified water.
What STALORAL looks like and what the pack contains
STALORAL is a clear, colorless or yellow-brown solution, depending on the type of allergen and its concentration.
The basic treatment kit contains:
2 vials of 10 ml (concentration: 10 IR/ml or 10 IC/ml; concentration: 100 IR/ml or 100 IC/ml) and 2 dosing pumps.
Blue cap concentration: 10 IR/ml or 10 IC/ml
Red cap
concentration: 100 IR/ml or 100 IC/ml
The maintenance treatment kit contains:
2 vials of 10 ml (concentration: 100 IR/ml or 100 IC/ml) - red caps.
2 dosing pumps.
Marketing authorization holder and manufacturer
STALLERGENES
6, rue Alexis de Tocqueville
92160 Antony, France
To obtain more detailed information, you should contact the representative of the marketing authorization holder in Poland:
STALLERGENES Sp. z o.o.
phone: 22 620 29 98
Date of last update of the leaflet:
STALORAL ANNEX 1
I. Plant-based allergen extracts
Weeds
641 Alfalfa (Medicago sativa)
IC/ml
664 Dandelion (Taraxacum vulgare)
IC/ml
623 Fat hen (Chenopodium album)
IC/ml
673 Goldenrod (Solidago canadensis)
IC/ml
636 Hop (Humulus lupulus)
IC/ml
605 Mugwort (Artemisia vulgaris)
IR/ml
646 Black mustard (Brassica nigra)
IC/ml
654 Nettle (Urtica dioica)
IC/ml
643 Oxeye daisy (Chrysanthemum leucanthemum)
IC/ml
665 Plantain (Plantago)
IC/ml
604 Ragweed (Ambrosia elatior)
IR/ml
625 Oilseed rape (Brassica oleifera)
IC/ml
710 Russian thistle (Salsola kali)
IC/ml
655 Sorrel (Rumex acetosa)
IC/ml
678 Sunflower (Helianthus annus)
IC/ml
657 Parietaria officinalis
IR/ml
Grasses
601 Red fescue (Agrostis vulgaris)
IC/ml
705 Bermuda grass (Cynodon dactylon)
IR/ml
627 Orchard grass (Dactylis glomerata)
IR/ml
624 Quackgrass (Agropyron repens)
IC/ml
630 Meadow fescue (Festuca elatior)
IC/ml
658 Kentucky bluegrass (Poa pratensis)
IR/ml
638 Perennial ryegrass (Lolium perenne)
IR/ml
631 Sweet vernal grass (Anthoxantum odoratum)
IR/ml
661 Timothy grass (Phleum pratense)
IR/ml
637 Velvet grass (Holcus lanatus)
IC/ml
Cereals
652 Barley (Hordeum vulgare)
IC/ml
642 Corn (Zea mays)
IC/ml
610 Oat (Avena sativa)
IC/ml
671 Rye (Secale cereale)
IR/ml
614 Wheat (Triticum vulgare)
IC/ml
106 Wheat flour
IC/ml
Trees
609 Alder (Alnus glutinosa)
IR/ml
632 Ash (Fraxinus excelsior)
IC/ml
635 Beech (Fagus sylvatica)
IC/ml
615 Birch (Betula alba)
IR/ml
620 Chestnut (Castanea vulgaris)
IC/ml
626 Cypress (Cupressus sempervirens)
IC/ml
675 Elder (Sambucus nigra)
IC/ml
653 Elm (Ulmus campestris)
IC/ml
667 Black locust (Robinia pseudoacacia)
IC/ml
649 Hazel (Corylus avellana)
IR/ml
619 Hornbeam (Carpinus betulus)
IR/ml
644 Horse chestnut (Aesculus hippocastanum)
IC/ml
634 Juniper (Juniperus communis)
IC/ml
677 Lime (Tilia platyphyllos)
IC/ml
629 Sycamore (Acer pseudoplatanus)
IC/ml
645 Silver fir (Abies alba)
IC/ml
647 White mulberry (Morus alba)
IC/ml
621 English oak (Quercus robur)
IC/ml
651 Olive (Olea europaea)
IR/ml
662 Scots pine (Pinus sylvestris)
IC/ml
666 Plane tree (Platanus vulgaris)
IC/ml
659 White poplar (Populus alba)
IC/ml
680 Privet (Ligustrum vulgare)
IC/ml
650 Walnut (Juglans regia)
IC/ml
669 Goat willow (Salix caprea)
IC/ml
Tobacco
904 Tobacco (leaves)
IC/ml
II. Animal-based allergen extracts(dander, epithelia, insects)
Animals
507 Cat
IR/ml
509 Dog
IC/ml
508 Goat
IC/ml
510 Guinea pig
IC/ml
511 Hamster
IC/ml
516 Horse
IC/ml
512 Rabbit
IC/ml
505 Sheep's wool
IC/ml
Insects
301 Cockroach
IC/ml
303 Grain weevil
IC/ml
310 Horsefly
IC/ml
307 Mosquito
IC/ml
III. House dust mite allergen extracts
325 Acarus siro
IC/ml
314 Dermatophagoides farinae
IR/ml
315 Dermatophagoides pteronyssinus
IR/ml
326 Euroglyphus maynei
IC/ml
324 Glyciphagus domesticus
IC/ml
317 Lepidoglyphus destructor
IC/ml
318 Tyrophagus putrescentiae
IC/ml
IV. Fungal allergen extracts(mold fungi, yeast, dermatophytes)
402 Botrytis cinerea
IC/ml
403 Candida albicans
IC/ml
407 Chaetomium globosum
IC/ml
409 Epicoccum
IC/ml
410 Epidermophyton
IC/ml
411 Fusarium
IC/ml
413 Helminthosporium
IC/ml
447 Merulius lacrymans
IC/ml
417 Mucor racemosus
IC/ml
425 Pullularia pullulans
IC/ml
426 Rhizopus nigricans
IC/ml
432 Stemphylium botryosum
IC/ml
435 Trichophyton
IC/ml
405 Trichothecium roseum (Cephalothecium)
IC/ml
V. Mixtures of allergen extracts * of animal origin
506 Feather mixture (duck, goose, chicken)
IC/ml
* of plant origin
a) weeds
719 Mixture I - Compositae (Goldenrod, Dandelion, Lampourde, Ox-eye-daisy) IC/ml
714 Mixture II - Chenopodiaceae (Fat hen, Rough pigweed)
IC/ml
706 Mixture III - Weed mixtures (Alfalfa, Red clover, Mustard, Nettle, Sorrel) IC/ml
b) grasses
701 3 grasses- (Orchard grass, Perennial ryegrass, Timothy grass)
IR/ml
688 5 grasses- (Orchard grass, Kentucky bluegrass, Perennial ryegrass, Sweet vernal grass, Timothy grass)
IR/ml
689 12 grasses- (Red fescue, Bermuda grass, Bromus, Orchard grass, Meadow fescue, Kentucky bluegrass, Oat grass, Perennial ryegrass, Sweet vernal grass, Timothy grass, Wild oat, Velvet grass)
IR/ml
690 5 grasses/4 cereals- (Orchard grass, Kentucky bluegrass, Perennial ryegrass, Sweet vernal grass, Timothy grass)/(Barley, Corn, Oat, Wheat)
IR/ml
687 4 cereals- (Barley, Corn, Oat, Wheat)
IR/ml
c) trees
702 Betulaceae (Alder, Birch, Hazel, Hornbeam)
IR/ml
696 Fagaceae (Beech, Chestnut, English oak)
IC/ml
716 Cupressaceae (Cypress, Juniper)
IC/ml
715 Oleaceae (Ash, Olive, Privet)
IC/ml
717 Salicaceae (Poplar, Willow)
IC/ml
718 Tree mixture (Sycamore, Horse chestnut, Plane tree, Black locust, Lime)
IC/ml
917 Woodworker's lung (English oak, Beech, Cherry, Scots pine)
IC/ml
* mites
350 D. pteronyssinus + D. farinae
IR/ml
330 Grain mite (Acarus siro, Glyciphagus domesticus, Lepidoglyphus destructor, Tyrophagus putrescentiae)
IC/ml
* fungi
400 Alternaria (Alternatalongipes)
IC/ml
414 Cladosporium (Cladosporoides, herbarum)
IC/ml
401 Aspergillus (Fumigatus, nidulans, niger)
IC/ml
422 Penicillium (Digitatum, expansum, notatum)
IC/ml
445 Yeast mixture (Saccharomyces cerevisiae, minor)
IC/ml
446 Smut mixture (Ustilago avenae, tritici, holci, zeae)
IC/ml
- Country of registration
- Prescription requiredYes
- Manufacturer
- ImporterStallergenes
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to StaloralDosage form: Lyophilizate, 12 SQ-HDMActive substance: house dust mitesManufacturer: ALK-Abello S.A.Prescription requiredDosage form: Tablets, 100 IRActive substance: house dust mitesManufacturer: Stallergenes S.A.S.Prescription requiredDosage form: Tablets, 100 IR+ 300 IRActive substance: house dust mitesManufacturer: Stallergenes S.A.S.Prescription required
Alternatives to Staloral in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Staloral in Ukraine
Alternative to Staloral in Spain
Online doctors for Staloral
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Staloral – subject to medical assessment and local rules.














