
Staloral 300

Ask a doctor about a prescription for Staloral 300

How to use Staloral 300
Leaflet attached to the packaging: patient information
STALORAL 300
Plant origin allergen extracts (plant pollen)
House dust mite allergen extracts
Allergen extract mixtures
Sublingual solution
10 IR/ml; 300 IR/ml
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist or nurse. See section 4.
Table of contents of the leaflet
- 1. What is STALORAL 300 and what is it used for
- 2. Important information before using STALORAL 300
- 3. How to use STALORAL 300
- 4. Possible side effects
- 5. How to store STALORAL 300
- 6. Contents of the packaging and other information
1. What is STALORAL 300 and what is it used for
Specific immunotherapy medicine. It is used in patients allergic to plant origin allergens (plant pollen) or house dust mites, whose list is attached to this leaflet.
Indications for use
Allergic diseases of a seasonal or year-round nature, manifested by rhinitis (sneezing, runny nose or itchy nose, stuffy nose), conjunctivitis (itchy and watery eyes) or asthma (mild or moderate) in adults and children (over 5 years old).
The goal of treatment with STALORAL 300 is to increase immunological tolerance to allergens, and thus alleviate allergic reactions.
2. Important information before using STALORAL 300
When not to use STALORAL 300
- if the patient has been found to be hypersensitive (allergic) to any of the other ingredients of STALORAL 300 (see section 6);
- if the patient has been found to have immune system disorders or diseases;
- if the patient has been found to have cancer;
- if the patient has been found to have severe or uncontrolled asthma;
- if the patient has been found to have oral thrush.
Only a doctor can determine if there are contraindications to the use of the medicine.
Warnings and precautions
Before starting treatment, symptoms of allergy should be stabilized with appropriate symptomatic treatment, if necessary.
Before starting treatment with STALORAL 300, the doctor or pharmacist should be informed:
- if the patient has severe clinical symptoms of allergy at the start of treatment - treatment should be postponed,
- if the patient has had other diseases recently or if the allergy has worsened recently,
- if the patient is to undergo oral surgery or have a tooth extracted. Treatment with STALORAL 300 should be discontinued until the oral cavity has fully healed,
- if the patient has had eosinophilic esophagitis in the past,
- if the patient experiences severe or persistent abdominal pain, difficulty swallowing, or chest pain during treatment,
- if the patient is on a low-sodium diet, as the medicine contains sodium (see "STALORAL 300 contains sodium chloride"),
- if the patient is taking beta-adrenergic blockers (including eye drops or ointments).
STALORAL 300 and other medicines
The doctor should be told about all medicines taken recently, including those available without a prescription.
Before starting treatment, the doctor should be informed:
- if the patient is taking certain antidepressants (tricyclic antidepressants or monoamine oxidase inhibitors - MAOIs), as there is a greater risk of side effects if epinephrine (prescribed in case of severe allergic reactions) is needed, even with a fatal outcome
- if the patient is scheduled for preventive vaccination against infectious diseases. Vaccination can be performed without interrupting treatment, but only after assessing the patient's overall health.
Symptomatic treatment (e.g., with antihistamines and/or nasal corticosteroids) may be used at the same time as STALORAL 300.
Pregnancy and breastfeeding
Pregnant women, or women who suspect they are pregnant, or plan to become pregnant, should consult their doctor or pharmacist before using this medicine.
Breastfeeding women should consult their doctor or pharmacist before using this medicine.
There is no experience with the use of STALORAL 300 in pregnant or breastfeeding women, so immunotherapy should not be started in pregnant or breastfeeding women, unless the doctor considers it necessary.
Women who become pregnant or start breastfeeding during treatment with STALORAL 300 should consult their doctor about continuing to take this medicine.
Driving and using machines
There are no contraindications to driving or using machines during treatment with STALORAL 300.
STALORAL 300 contains sodium chloride.
In the case of patients on a low-sodium diet, the presence of sodium chloride in the medicine should be taken into account (one 10 ml vial contains 590 mg of sodium chloride). See "Warnings and precautions".
3. How to use STALORAL 300
Recommended dose
STALORAL 300 should always be used in accordance with the doctor's recommendations. In case of doubts, you should contact your doctor or pharmacist again. In the event of using a larger dose of STALORAL 300 than recommended or missing a dose of STALORAL 300,
you should read the information in the relevant sections of the leaflet.
Specific immunotherapy is most effective when started in the early stages of the disease.
The dose size does not depend on age, but on the degree of individual patient reactivity.
In the case of seasonal allergies, it is recommended to start treatment before the pollen season and continue until the end of the pollen season.
In the case of year-round allergies, it is recommended to continue treatment throughout the year.
Treatment is divided into two stages:
- basic treatment - with gradual dose increase
- maintenance treatment - using a constant dose.
In treatment, allergen solutions of different concentrations are used, for which the packaging differs in cap color:
Cap color: blue - concentration 10 IR/ml
Cap color: purple - concentration 300 IR/ml
Basic treatment with dose increase
The medicine dose should be measured under the tongue by pressing the pump dispenser and after 2 minutes it should be swallowed.
The medicine is used daily, with each dose increased until the maintenance dose is reached according to the following scheme:
| First week | Second week | ||||
| Concentration: 10 IR/ml | Concentration: 300 IR/ml | ||||
| Day | number of presses | Dose (IR) | Day | number of presses | Dose (IR) |
| 1 2 3 4 5 | 1 2 3 4 5 | 2 4 6 8 10 | 6 7 8 9 | 1 2 3 4 | 60 120 180 240 |
The above dosing scheme should be considered as a guideline and example of treatment. Treatment can be modified depending on the patient's condition and their reaction to treatment.
Maintenance treatment - constant dose
The maximum tolerated dose should be taken daily or three times a week.
The recommended dosing of the medicine is at least 4 presses 3 times a week or 2 to 4 presses taken daily, using a solution with a concentration of 300 IR/ml.
Clinical trials with the use of STALORAL 300 with various allergens have shown that a daily dose of 300 IR is well tolerated.
Duration of treatment
Usually, desensitization treatment is used for a period of 3 to 5 years.
The doctor will reassess the treatment in case of no significant improvement in symptoms after a year (year-round allergy) or after the first pollen season (seasonal allergy).
Method of administration
To ensure safe use of the medicine, the doctor's instructions should be followed strictly.
STALORAL 300 is administered sublingually.
Before taking the medicine, the information on the packaging should be carefully checked and it should be ensured that the data is consistent with the doctor's recommendation (prescription): patient's name, medicine composition and concentration, as well as the expiry date of the medicine.
The medicine should be taken during the day, to an empty stomach, without food and drink.
The medicine dose should be measured under the tongue by pressing the pump dispenser and after 2 minutes it should be swallowed.
The doctor may recommend a different dosing schedule depending on the patient's condition and their reaction to treatment.
STALORAL 300 should be administered to children by adults.
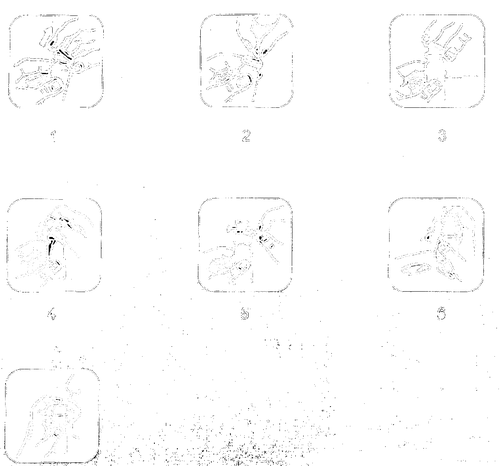
Instructions for the first use of the medicine:
During the first use, the following points should be followed:
- 1. Remove the plastic, colored part of the cap.
- 2. Pull the metal tab and completely remove the aluminum cap.
- 3. Remove the gray plug.
- 4. Remove the pump dispenser from the plastic cover. Place the vial on a flat surface, and then, holding it firmly with one hand, press the pump dispenser firmly into place.
- 5. Remove the purple safety ring.
- 6. Pump, repeating the presses of the pump dispenser. After 5 presses, the pump dispenser measures 1 full dose of the medicine.
- 7. Place the tip of the pump dispenser in the mouth, directly under the tongue. Press firmly to obtain a full dose. Repeat the action to obtain the number of doses prescribed by the doctor. Hold the solution under the tongue for 2 minutes and swallow.
- 8. After use, clean the tip of the pump dispenser and put the protective ring back on.
- 9. For subsequent uses, remove the protective ring and repeat steps 7 and 8.
7
Usually, desensitization treatment is used for 3 to 5 years. In the case of seasonal allergies, specific immunotherapy can be carried out for several seasons in subsequent years.
Use in children
Allergen immunotherapy is not recommended in children under 5 years old.
Using a larger dose of STALORAL 300 than recommended
The doctor or pharmacist should be told immediately.
In the event of using a larger dose of the medicine than recommended, the risk of side effects increases or their severity may increase.
Missing a dose of STALORAL 300
A double dose should not be used to make up for a missed dose.
Treatment should be continued the next day, taking the established dose.
Stopping treatment with STALORAL 300
If the treatment with STALORAL 300 is discontinued for less than a week, treatment can be continued using the last dose. If treatment is discontinued for more than a week, the doctor should be consulted.
In case of any further doubts related to the use of this medicine, the doctor or pharmacist or nurse should be consulted.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
During treatment with STALORAL 300, the patient will be exposed to the action of substances that can cause allergic reactions at the injection site and/or symptoms that can affect the whole body.
Such reactions may occur at the start of treatment or later, during treatment.
The doctor should be told immediately and medical help should be sought in case of the following symptoms:
Severe allergic reaction with rapid onset of symptoms, affecting the whole body, e.g. intense itching or rash, difficulty breathing, abdominal pain, or symptoms related to low blood pressure, such as dizziness, malaise.
Tolerance to a given dose may change over time, depending on the patient's health and environment.
In case of side effects, the doctor should be consulted, who may change the treatment schedule.
The doctor may prescribe pre-treatment with anti-allergic medicines to reduce the frequency and severity of side effects.
Additional side effects
Frequent (affecting less than 1 in 10 patients)
Mouth disorders (e.g., swelling, discomfort, tingling, itching, numbness, blisters, ulcers), tongue swelling, discomfort in the throat or pain or swelling or irritation,
rhinitis (stuffy nose, runny nose, sneezing, itchy nose, discomfort in the nose), cough,
salivary gland disorders, itchy eyes, itchy ears, nausea, vomiting, abdominal pain, diarrhea, redness or itching of the skin.
Uncommon (affecting less than 1 in 100 patients)
Eye disorders (redness, irritation of the eyes, watery eyes), herpes, hoarseness, difficulty breathing, asthma, hives, atypical skin allergy symptoms (burning, tingling, pricking), gastritis, esophageal spasms.
Rare (affecting less than 1 in 1000 patients)
Headache, eczema, joint pain, muscle pain, weakness, enlarged lymph nodes (glands), fever.
The following side effects have also been reported:
Taste disorders, dry mouth, dizziness, facial swelling, severe allergic reactions, esophagitis.
Reporting side effects
If side effects occur, including any side effects not listed in the leaflet, the doctor or pharmacist or nurse should be told. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel. 22 49 21 301, fax 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store STALORAL 300
The medicine should be stored out of sight and reach of children.
Before opening: Store in a refrigerator (2°C - 8°C).
After opening:
10 IR/ml: Store in a refrigerator (2°C - 8°C) for up to 30 days.
300 IR/ml: Store at a temperature below 25°C for up to 30 days or in a refrigerator (2°C - 8°C) for up to 30 days.
During transport, vials should always be carried in an upright position.
The medicine should not be used for more than 30 days after opening and after the expiry date stated on the packaging after the "EXP" date. The expiry date refers to the last day of the given month.
Medicines should not be disposed of via wastewater or household waste. The pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What does STALORAL 300 contain
The active substance of the medicine is allergen extracts or their mixtures at a concentration of 10 or 300 IR/ml (according to Annex 1) in combination with mannitol. The amount of mannitol does not exceed 40 mg/ml.
The qualitative composition of the active substance may be individually selected for the patient within the range of allergens listed in Annex 1.
The activity of the relevant allergen expressed in IR units/ml is given on the label.
* IR (reactivity index): the allergen extract has an activity equal to 100 IR/ml if it causes a wheal with a diameter of 7 mm in a prick test using a Stallerpoint needle in 30 people allergic to the given allergen.
Additionally, the sensitivity of the tested persons is confirmed by a positive reaction to 9% codeine phosphate or 10 mg/ml histamine dihydrochloride in a prick test.
Other ingredients are: sodium chloride, glycerol, purified water
What does STALORAL 300 look like and what does the packaging contain
Basic treatment set:
3 vials of 10 ml (concentration 10 IR/ml, concentration 300 IR/ml, concentration 300 IR/ml)
+ 3 pump dispensers
Maintenance treatment set:
2 vials of 10 ml (concentration 300 IR/ml, concentration 300 IR/ml)
+ 2 pump dispensers
Marketing authorization holder and manufacturer
STALLERGENES
6, rue Alexis de Tocqueville
92160 Antony, France
For more detailed information, please contact the representative of the marketing authorization holder in Poland:
STALLERGENES Sp. z o.o.
tel. 22 620 29 98
Date of last update of the leaflet: 02/2024
STALORAL 300
ANNEX 1
I. PLANT ORIGIN ALLERGEN EXTRACTS (PLANT POLLEN)
Grasses and cereals:
661Timothy grass (Phleum pratense)
Trees:
609Black alder (Alnus glutinosa)
615White birch (Betula alba)
651European olive (Olea europea)
649Hazel (Corylus avellana)
II. HOUSE DUST MITE ALLERGEN EXTRACTS
315Dermatophagoides pteronyssinus
314Dermatophagoides farinae
III. ALLERGEN EXTRACT MIXTURES
Of plant origin:
6885 grasses (meadow fescue, sweet vernal grass, perennial ryegrass, meadow grass, timothy grass)
Of house dust mites:
350Dermatophagoides pteronyssinus, Dermatophagoides farinae
- Country of registration
- Prescription requiredYes
- Manufacturer
- ImporterStallergenes
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Staloral 300Dosage form: Lyophilizate, 12 SQ-HDMActive substance: house dust mitesManufacturer: ALK-Abello S.A.Prescription requiredDosage form: Tablets, 100 IRActive substance: house dust mitesManufacturer: Stallergenes S.A.S.Prescription requiredDosage form: Tablets, 100 IR+ 300 IRActive substance: house dust mitesManufacturer: Stallergenes S.A.S.Prescription required
Alternatives to Staloral 300 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Staloral 300 in Ukraine
Alternative to Staloral 300 in Spain
Online doctors for Staloral 300
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Staloral 300 – subject to medical assessment and local rules.














