
How to use Soolantra
Package Leaflet: Information for the Patient
Warning! Keep the Leaflet! Information on the Immediate Packaging in a Foreign Language.
Soolantra
10 mg/g, Cream
Ivermectin
Read the Leaflet Carefully Before Using the Medication, as it Contains Important Information for the Patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medication has been prescribed to a specific person. Do not pass it on to others. The medication may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Soolantra and what is it used for
- 2. Important information before using Soolantra
- 3. How to use Soolantra
- 4. Possible side effects
- 5. How to store Soolantra
- 6. Contents of the packaging and other information
1. What is Soolantra and what is it used for
Soolantra contains the active substance ivermectin, which belongs to the avermectin group of medications.
This medication is applied to the skin to treat papules and pustules in rosacea.
Soolantra can only be used in adults (18 years or older).
2. Important information before using Soolantra
When not to use Soolantra:
- if the patient is allergic to ivermectin or any of the other ingredients of this medication (listed in section 6).
Warnings and precautions
Before starting to use Soolantra, discuss it with your doctor or pharmacist.
At the beginning of treatment, some patients may experience worsening of rosacea symptoms,
however, this is not common and usually resolves within the first week of treatment. If worsening of rosacea symptoms occurs, inform your doctor.
Soolantra and other medications
Other medications may affect Soolantra; therefore, inform your doctor about all medications currently being used or recently used, as well as any medications planned to be used.
Pregnancy and breastfeeding
Soolantra should not be used during pregnancy.
If the patient is breastfeeding, they should not use this medication; alternatively, breastfeeding can be stopped before starting treatment with Soolantra. Consult a doctor to decide whether to use the medication or breastfeed, considering the benefits of treatment and the benefits of breastfeeding.
Driving and operating machinery
Soolantra has no or negligible influence on the ability to drive and use machines.
What Soolantra contains:
- cetyl alcohol and stearyl alcohol, which may cause skin reactions (e.g., contact dermatitis),
- methyl parahydroxybenzoate (E 218) and propyl parahydroxybenzoate (E 216), which may cause allergic reactions (also late-type),
- propylene glycol, which may cause skin irritation.
3. How to use Soolantra
Always use this medication exactly as your doctor has told you.
In case of doubts, consult a doctor or pharmacist.
Important information:Soolantra is for use on the face only in adults. Do not use this medication on other parts of the body, especially on moist surfaces, such as the eyes, mouth, or any mucous membrane. Do not swallow.
The recommended dose is one application to the face per day. Apply a pea-sized amount to each of the five areas of the face: forehead, chin, nose, and each cheek. Then spread the cream in a thin layer over the entire face.
Do not apply the cream near the eyes, mouth, or any mucous membrane, for example, inside the nose, mouth, or eyes. If the cream accidentally gets into the eyes or around the eyes, rinse the area with plenty of water.
Do not use cosmetics (such as facial creams or other makeup products) before daily application of Soolantra. These products can be applied after the applied cream has dried.
Wash your hands immediately after applying the cream.
Use Soolantra every day for the entire treatment period; the treatment cycle can be repeated.
Your doctor will tell you how long to use Soolantra. The treatment duration may vary from person to person and depends on the severity of skin lesions.
Improvement can be observed after 4 weeks of treatment. If there is no improvement after 3 months, stop using Soolantra and consult a doctor.
Liver function disorders
If the patient has liver problems, consult a doctor before using Soolantra.
Using Soolantra in children and adolescents
Do not use Soolantra in children and adolescents.
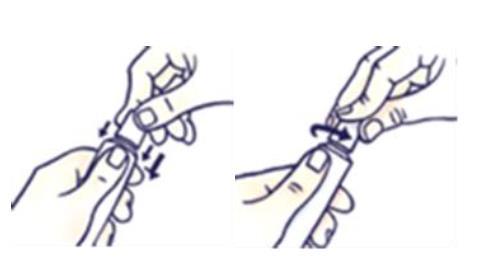
How to open the tube with a child-resistant cap
To avoid spilling the medication, do not squeeze the tube while opening or closing it.
Press the cap and turn it counterclockwise (to the left).
Then remove the cap.
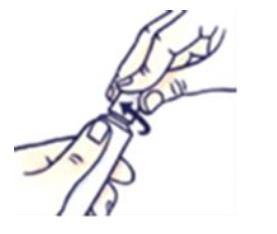
How to close the tube with a child-resistant cap
Press the cap and turn it clockwise (to the right).
Using a higher dose of Soolantra than recommended
In case of using a dose higher than the recommended daily dose, contact a doctor who will advise on what actions to take.
Missing a dose of Soolantra
Do not use a double dose to make up for a missed dose.
Stopping the use of Soolantra
Papules and pustules will decrease only after several applications of this medication. It is important to continue using Soolantra as long as the doctor recommends.
In case of any further doubts about using this medication, consult a doctor or pharmacist.
4. Possible side effects
Like all medications, Soolantra can cause side effects, although not everybody gets them.
Soolantra may cause the following side effects:
Common side effects (may affect up to 1 in 10 people):
- burning sensation of the skin.
Uncommon side effects (may affect up to 1 in 100 people):
- skin irritation
- skin itching
- skin dryness
- worsening of rosacea symptoms (consult a doctor).
Side effects of unknown frequency (cannot be estimated from the available data):
- skin redness
- skin inflammation
- facial swelling
- increased liver enzyme activity (AlAT/AspAT).
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, inform a doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, more information can be collected on the safety of the medication.
5. How to store Soolantra
Keep the medication out of the sight and reach of children.
Do not use this medication after the expiry date stated on the packaging.
The expiry date is the last day of the month stated.
Shelf life after first opening the tube: 6 months.
No special precautions for storage.
Medications should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medications that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Soolantra contains
- The active substance of Soolantra is ivermectin. One gram of cream contains 10 mg of ivermectin.
- The other ingredients are: glycerol, isopropyl palmitate, carbomer, dimethicone, disodium edetate, citric acid monohydrate, cetyl alcohol, stearyl alcohol, macrogol cetostearyl ether, sorbitan stearate, methyl parahydroxybenzoate (E 218), propyl parahydroxybenzoate (E 216), phenoxyethanol, propylene glycol, oleyl alcohol, sodium hydroxide, purified water.
What Soolantra looks like and what the packaging contains
Soolantra is a white to light yellow cream.
Pack size:
Tube containing 30 g of cream, with a child-resistant closure, in a cardboard box.
For more detailed information, consult the marketing authorization holder or parallel importer.
Marketing authorization holder in the Czech Republic, the country of export:
Galderma International
Tour Europlaza, 20 avenue André Prothin, La Défense 4
92927 La Défense Cedex, France
Manufacturer:
Laboratoires Galderma
- Z. l. Montdésir 74540 Alby-sur-Chéran, France
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Marketing authorization number in the Czech Republic, the country of export:46/241/15-C
Parallel import authorization number: 187/25
This medicinal product has been authorized in the EEA Member States under the following names:
Austria, Germany, Portugal:
Soolantra 10 mg/g Cream
Belgium, Luxembourg:
Soolantra 10 mg/g crème
Soolantra 10 mg/g Cream
Bulgaria:
Soolantra 10 mg/g Крем
Cyprus, Greece:
Soolantra 10 mg/g Κρέμα
Czech Republic, Hungary, Slovakia:
Soolantra 10 mg/g krém
Denmark:
Soolantra
Estonia:
Soolantra 10 mg/g kreem
Finland:
Soolantra 10 mg/g emulsiovoide
France, Netherlands:
Soolantra 10 mg/g crème
Iceland, Norway, Poland:
Soolantra 10 mg/g krem
Ireland, United Kingdom:
Soolantra 10 mg/g cream
Italy:
Efacti 10 mg/g Crema
Latvia:
Soolantra 10 mg/g krēms
Lithuania:
Soolantra 10 mg/g kremas
Malta:
Soolantra 10 mg/g krema
Romania:
Soolantra 10 mg/g Cremă
Spain:
Soolantra 10 mg/g crema
Sweden:
Soolantra 10 mg/g kräm
Date of approval of the leaflet: 23.05.2025
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Galderma International
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SoolantraDosage form: Cream, 10 mg/gActive substance: ivermectinManufacturer: Laboratoires Galderma S.A.Prescription requiredDosage form: Cream, 10 mg/gActive substance: ivermectinPrescription requiredDosage form: Solution, 20 mg/mlActive substance: minoxidilManufacturer: Industrial Farmaceutica Cantabria, S.A.Prescription not required
Alternatives to Soolantra in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Soolantra in Ukraina
Alternative to Soolantra in Hiszpania
Online doctors for Soolantra
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Soolantra – subject to medical assessment and local rules.







