
Sativex

Ask a doctor about a prescription for Sativex

How to use Sativex
PATIENT INFORMATION LEAFLET
Leaflet accompanying the packaging: patient information
Sativex, oral spray
delta-9-tetrahydrocannabinol+ cannabidiol
You should read the contents of this leaflet before using the medicine, as it contains important information for you.
- You should keep this leaflet, so you can read it again later.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- 1. What Sativex is and what it is used for
- 2. Important information before using Sativex
- 3. How to use Sativex
- 4. Possible side effects
- 5. How to store Sativex
- 6. Contents of the pack and other information
1. What Sativex is and what it is used for
Sativex is an oral spray that contains extracts from Cannabiscalled cannabinoids.
What Sativex is used for
Sativex is used for the treatment of spasticity in multiple sclerosis (MS). This spasticity can cause your muscles to feel stiff, making it harder to move than normal.
Four-week trial of Sativex
Only a specialist doctor can start you on Sativex treatment.
- Before starting treatment with Sativex, your specialist will assess your muscle spasticity and check if any other treatments you are taking have worked.
- Then you will try Sativex for 4 weeks. After this, your doctor will assess you again to see if Sativex is helping you.
- Only if you have shown a significant improvement in spasticity after 4 weeks can you continue to take Sativex.
2. Important information before using Sativex
When not to use Sativex:
- If you are allergic to Cannabisextracts or any of the other ingredients of this medicine (listed in section 6).
- If you or a close relative have ever had any mental health problems, such as schizophrenia or psychosis.
or other significant mental problems. This does not include depression associated with multiple sclerosis.
- If you are breastfeeding.
Do not use this medicine if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before using Sativex.
Warnings and precautions
Before starting treatment with Sativex, tell your doctor or pharmacist if:
- You are pregnant or planning to have a baby.
- You are under 18 years old.
- You have epilepsy or regular fits (seizures).
- You have kidney problems.
- You have moderate or severe liver problems.
- You have serious heart problems, such as angina, poorly controlled high blood pressure or heart rhythm problems, or have had a heart attack.
- You are elderly, especially if you have problems carrying out everyday activities, such as preparing hot meals and drinks.
- You have previously abused drugs or alcohol.
Regardless of whether you are male or female, you must use effective contraception while using this medicine [see also below “Pregnancy, breastfeeding and contraception (men and women)”].
If any of the above apply to you (or if you are not sure), talk to your doctor or pharmacist before using Sativex.
Sativex with other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and any you plan to take.
- medicines for anxiety or sleep problems (such as benzodiazepines, e.g. diazepam, triazolam, or other sedatives, e.g. zopiclone, zolpidem, buspirone, St John’s Wort)
- medicines for increased muscle tension (such as baclofen)
- antibiotics (such as rifampicin, clarithromycin)
- medicines for epilepsy or nerve pain (such as phenytoin, phenobarbital, carbamazepine)
- medicines for high cholesterol (such as statins, e.g. atorvastatin, simvastatin)
- medicines for fungal infections (such as itraconazole, fluconazole, and ketoconazole)
- medicines for HIV (such as ritonavir)
- blood thinners (such as warfarin)
- medicines for heart problems (such as beta blockers, e.g. bisoprolol, propranolol)
- corticosteroids (such as hydrocortisone, beclometasone, prednisolone)
- hormonal contraceptives or treatments for certain types of cancer (such as ethinylestradiol, levonorgestrel, or dydrogesterone)
- anaesthetics (such as propofol) used before surgery.
If any of the above apply to you (or if you are not sure), talk to your doctor or pharmacist before using Sativex.
Sativex with food, drink, and alcohol
- While using Sativex, you should avoid drinking alcohol, especially at the start of treatment or when changing your dose. If you drink alcohol while using Sativex, be aware that using Sativex and drinking alcohol can increase the effects of both the medicine and the alcohol (such as loss of balance or reaction time), which can increase the risk of falls and other accidents.
- Sativex can be used with or without food. However, taking Sativex with food may affect how much medicine is absorbed by your body. Try to use Sativex in the same way each day in relation to food, so you get the same effect each time.
Pregnancy, breastfeeding, and contraception (men and women)
- If you are pregnant or think you may be pregnant, or if you plan to have a baby, ask your doctor or pharmacist for advice before using this medicine.
- Do not use Sativex if you are pregnant, unless your doctor advises you to.
- Sativex may reduce the effectiveness of hormonal contraceptives, such as the pill or implants. This means you should use an additional method of contraception. Whether you are male or female, you must use a reliable method of barrier contraception, such as a condom, diaphragm, or cap, while using this medicine. You should continue to use contraception for at least 3 months after stopping treatment.
- Do not use Sativex if you are breastfeeding.
Driving and using machines
- Do not drive or operate machinery from the time you first start using Sativex until you know how the medicine affects you.
- Sativex can cause drowsiness or dizziness, which may affect your ability to judge your surroundings or react quickly. There have also been rare reports of loss of consciousness.
- Once you are used to taking Sativex and your dose has been decided, you should still not drive or operate machinery if Sativex causes side effects such as drowsiness or dizziness that could affect your ability to do so. If you are in any doubt, do not drive or operate machinery.
You should talk to your doctor or pharmacist if you are not sure.
Travelling abroad with Sativex
- Before travelling abroad, check if it is legal to take Sativex with you. This applies to any country you visit or travel through.
- Sativex is a controlled drug and its legal status varies between countries.
- In some countries, driving while using Sativex may be against the law.
Sativex contains ethanol and propylene glycol
- Sativex contains up to 40 mg of ethanol (alcohol) per single dose. The amount of alcohol in the maximum recommended daily dose (12 sprays) is equivalent to less than 2 teaspoons (10 ml) of beer or 1 teaspoon (5 ml) of wine. The small amount of alcohol in this medicine will not have any noticeable effects.
- Sativex contains propylene glycol, which may cause irritation. Each 100 microlitres of spray contains 52 mg of propylene glycol.
3. How to use Sativex
This medicine should always be used exactly as described in this leaflet or as advised by your doctor. If you are not sure, ask your doctor or pharmacist.
Sativex can only be used in the mouth - on the inside of the cheek or under the tongue.
- Sativex can be used with or without food. However, taking Sativex with food may affect how much medicine is absorbed by your body. Try to use Sativex in the same way each day in relation to food, so you get the same effect each time.
Opening the spray and preparing for use
- 1. Remove the spray from the refrigerator (see section 5 for important information about storing Sativex).
- 2. Write the date you open the spray on the label provided on the end of the leaflet. Stick the label on the spray container, so you can check the date. Do not use the spray after more than 6 weeks (42 days) from the date you open it.
- 3. Gently shake the spray container before use.
- 4. Remove the protective cap.
- 5. Hold the spray container between your thumb and index finger. Place your middle finger on the bottom of the spray nozzle.
- 6. Hold the spray container upright and press the spray nozzle 2-3 times to prepare the pump and ensure it works properly.
- 7. The spray is now ready to use. You do not need to prepare the pump again until you open a new spray container.
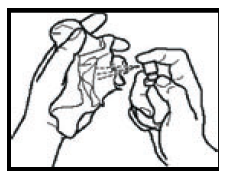
Using the spray
- 1. Hold the spray container between your thumb and index finger. Place your middle finger on the bottom of the spray nozzle.
- 2. Hold the spray container upright and point the nozzle towards your mouth. Direct the nozzle under your tongue or to the inside of your cheek. Change the area you spray in your mouth each time you use the spray. This helps prevent discomfort in one area.
- 3. Gently press the nozzle to release one spray. Do not use more than one spray at a time, even if you think you have only released a small amount of spray.
- 4. Replace the protective cap on the spray container.
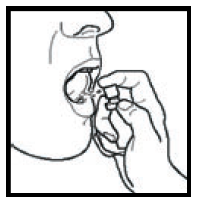
If you accidentally spray Sativex into your eyes, rinse them with water.
- Do not inhale the spray.
- Do not spray near children or pets.
- Do not use the spray near an open flame or heat source.
How to calculate the number of sprays to use
The number of sprays you need each day is individual and depends on you. Everyone needs a different number of sprays to get the most relief from muscle spasticity, with the fewest side effects.
- When you first start using Sativex, you should follow the schedule of days and times shown in the table below, until you find the number of sprays that works best for you.
- Stop increasing the number of sprays when you find the number that works best for you.This may take a few days or up to 2 weeks. You should then use this number of sprays each day. You can spread the number of sprays evenly throughout the day.
- Do not use more than one spray at a time. Always leave at least 15 minutes between sprays.
- Do not overexert yourself during the first few days of using Sativex, until you know how the medicine affects you.
- If you get side effects (usually dizziness), reduce the number of sprays you use each day by one spray, until you get the most relief from your symptoms, with the fewest side effects.
- Once you have found the number of sprays that works best for you, you should use this number of sprays each day. You can spread the number of sprays evenly throughout the day, in a way that suits you. Continue to leave at least 15 minutes between sprays.
| Number of sprays | |||
| Morning (between waking and noon) | Evening (between 4 pm and bedtime) | Total number of sprays per day | |
| Day 1 | 0 | 1 | 1 |
| Day 2 | 0 | 1 | 1 |
| Day 3 | 0 | 2 | 2 |
| Day 4 | 0 | 2 | 2 |
| Day 5 | 1 | 2 | 3 |
| Day 6 | 1 | 3 | 4 |
| Day 7 | 1 | 4 | 5 |
| Day 8 | 2 | 4 | 6 |
| Day 9 | 2 | 5 | 7 |
Do not use more than 12 sprays per day, unless your doctor advises you to.
Using more Sativex than prescribed
If you accidentally use more of this medicine than you should, you may:
- see or hear things that are not there (hallucinations);
- feel dizzy, sleepy, or disoriented;
- have changes in your heart rate.
- if you use more Sativex than you should, tell your doctor or pharmacist.
Missing a dose of Sativex
- If you miss a single dose, use the spray as soon as you remember or when you need to.
- Do not use 2 sprays at the same time to make up for a missed spray.
Knowing when the spray container is nearly empty
After the 3 preparation sprays, the spray container contains up to 90 doses of medicine. When the spray container is empty, the sound of the spray may change. You may also notice other changes when you spray the medicine in your mouth. This means the spray container is nearly empty. You should then open a new spray container.
Stopping treatment with Sativex
If for any reason you stop using Sativex, you should tell your doctor or pharmacist. If you stop using Sativex suddenly, you may have a short-term effect on your sleep, appetite, and mood. After stopping Sativex, muscle spasticity will usually return gradually.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you get any of the following serious side effects, stop using Sativex and contact your doctor or go to hospital straight away, as you may need to be monitored:
- seeing or hearing things that are not there (hallucinations).
- believing things that are not true.
- feeling that other people are against you.
- having thoughts of suicide.
- feeling depressed or disoriented.
- feeling extremely excited or detached from reality.
These side effects are more likely to happen at the start of treatment. In most cases, side effects are mild and usually go away within a few days.
- If you get any of the following side effects, use fewer sprays or stop using Sativex until you feel better.
| Day 10 | 3 | 5 | 8 |
| Day 11 | 3 | 6 | 9 |
| Day 12 | 4 | 6 | 10 |
| Day 13 | 4 | 7 | 11 |
| Day 14 | 5 | 7 | 12 |
- When you restart the medicine, go back to the number of sprays you were using that did not cause side effects.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Very common (affects more than 1 in 10 people)
- Dizziness or tiredness.
Common (affects less than 1 in 10 people)
- Memory or concentration problems.
- Drowsiness or mild dizziness.
- Blurred vision.
- Speech problems.
- Increased or decreased appetite.
- Changes in taste or dry mouth.
- Constipation or diarrhoea.
- Feeling sick (nausea) or being sick (vomiting).
- Mouth ulcers or pain, burning or inflammation in the mouth.
- Lack of energy or feeling weak or unwell.
- Feeling strange or drunk.
- Losing balance or falling.
Uncommon (affects less than 1 in 100 people)
- Fainting.
- Changes in heart rate, heart rhythm, or blood pressure.
- Sore throat or throat irritation.
- Stomach pain.
- Discoloration in the mouth or changes in tooth colour.
- Irritation at the site where Sativex is sprayed.
- Redness and swelling in the mouth or peeling of the lining of the mouth. Do not spray Sativex onto these areas.
Reporting side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in the https://smz.ezdrowie.gov.plwebsite.
5. How to store Sativex
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after “EXP”. The expiry date refers to the last day of that month.
- Store unopened Sativex containers in a refrigerator (2°C - 8°C). Sativex that is not stored in a refrigerator will lose its properties and should not be used again.
- After opening, store Sativex in an upright position at a temperature below 25°C.
- Do not use Sativex after 42 days from the date of opening the container.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Sativex contains
- The active substances are Cannabisextracts. 1 ml contains 38-44 mg and 35-42 mg of two extracts of Cannabis sativaL., leaf and flower, corresponding to 27 mg/ml delta-9-tetrahydrocannabinol (THC) and 25 mg/ml cannabidiol (CBD). Each 100 microlitres of spray contains 2.7 mg of THC and 2.5 mg of CBD.
- The other ingredients are anhydrous ethanol, propylene glycol, and peppermint oil.
What Sativex looks like and contents of the pack
Sativex is a yellow-brown liquid in a spray container with a pump and dose indicator, with a capacity of 10 ml. The pump is protected by a plastic cap.
The spray container contains up to 90 doses of medicine (after the 3 preparation sprays).
Sativex is packaged in cartons containing one or 2, 3, 4, 5, 6, 10, or 12 spray containers.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Jazz Pharmaceuticals Ireland Ltd
5th Floor
Waterloo Exchange
Waterloo Road
Dublin
D04 E5W7
Ireland
Manufacturer
GW Pharma (International) B.V.
Databankweg 26
3821AL Amersfoort
Netherlands
For more information about this medicine, contact the Marketing Authorisation Holder:
Almirall Sp. z o. o.
ul. Pileckiego 63
02-781 Warszawa
tel.: 22 330 02 57
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGW Pharma (International) B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SativexDosage form: Tablets, 30 mgActive substance: nefopamManufacturer: Holsten Pharma GmbH Laboratories BTTPrescription requiredDosage form: Tablets, 30 mgActive substance: nefopamManufacturer: Przedsiębiorstwo Farmaceutyczne Jelfa S.A.Prescription requiredDosage form: Liquid, 99.9 %Active substance: methoxyfluraneManufacturer: MIAS Pharma LimitedPrescription required
Alternatives to Sativex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sativex in Hiszpania
Online doctors for Sativex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sativex – subject to medical assessment and local rules.














