

Penthrox

Ask a doctor about a prescription for Penthrox

How to use Penthrox
Package Leaflet: Information for the User
Penthrox, 99.9%, Solution for Inhalation
Methoxyflurane
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you, do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist (see section 4).
Contents of the Package Leaflet
- 1. What is Penthrox and what is it used for
- 2. Important information before using Penthrox
- 3. How to use Penthrox
- 4. Possible side effects
- 5. How to store Penthrox
- 6. Contents of the pack and other information
1. What is Penthrox and what is it used for
Penthrox contains the active substance methoxyflurane. The medicine is indicated for the emergency relief of pain and is inhaled into the lungs through a single-use Penthrox inhaler. Penthrox is intended to reduce the severity of pain, not to completely eliminate it.
2. Important information before using Penthrox
When not to use Penthrox:
- or if your relatives have experienced severe side effects after using inhaled anesthetic agents;
Penthrox must not be used as an anesthetic agent. If you have any doubts about whether this medicine is suitable for you, consult your doctor or pharmacist.
Warnings and precautions
In the case of patients who are affected by the following points, the use of Penthrox should be discussed with a doctor before starting treatment:
- patients with kidney or liver disease;
- patients with conditions that may cause kidney problems;
- elderly patients.
Depression of breathing has been reported in patients treated with Penthrox, with symptoms such as slow and shallow breathing or other breathing difficulties (section 4). If breathing problems occur, you should immediately inform a medical professional.
Use in children and adolescents
Penthrox should not be used in children and adolescents under 18 years of age.
Penthrox with other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and about any medicines you plan to take, especially those listed below:
- Isoniazid used to treat tuberculosis.
- Phenobarbital or carbamazepine used to treat epilepsy.
- Rifampicin or other antibiotics used to treat infections.
- Medicines or substances that have a sedative effect on the nervous system, such as narcotics, painkillers, sedatives, sleeping pills, general anesthetics, phenothiazine, anxiolytics, muscle relaxants, and antihistamines with a sedative effect.
- Antibiotics and other medicines that may have a harmful effect on the kidneys, such as tetracycline, gentamicin, colistin, polymyxin B, and amphotericin, as well as contrast agents.
- Efavirenz or nevirapine used to treat HIV.
In case of doubts, consult your doctor. If you require hospital treatment with general anesthesia, you should inform the medical staff about the previous use of Penthrox.
Penthrox with food, drink, and alcohol
Do not drink alcohol while using the medicine, as it may enhance the effect of the medicine. You can take food and drinks while using the medicine, unless your doctor or pharmacist decides otherwise.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before using this medicine. Your doctor or pharmacist will discuss with you the possible risks and benefits of using Penthrox.
Driving and using machines
The medicine may affect your ability to drive and use machines. Before driving or using machines, make sure you are able to perform these activities. Penthrox may cause drowsiness and dizziness.
Penthrox contains butylhydroxytoluene (E 321)
Penthrox contains the excipient butylhydroxytoluene (E 321) (0.01% w/w). Butylhydroxytoluene may cause local skin reactions (e.g., contact dermatitis) or eye and mucous membrane irritation.
3. How to use Penthrox
This medicine should always be used as directed by your doctor. In case of doubts, consult your doctor or pharmacist. Adults: A single dose may consist of one or two bottles containing 3 ml of Penthrox. The maximum dose is two bottles containing 3 ml of Penthrox. Do not exceed the maximum dose of 6 ml.
Instructions for using Penthrox

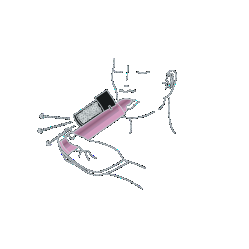
- 1. The doctor or other medical staff will prepare the inhaler for use and place a security strap on the patient's wrist.
- 2. The medicine should be inhaled through the inhaler mouthpiece to reduce the severity of pain. In case of doubts, ask the medical staff for help. To get used to the fruity smell of the medicine, the first few breaths should be taken cautiously. Exhale also through the inhaler. After cautious initial breaths, start breathing normally through the inhaler.
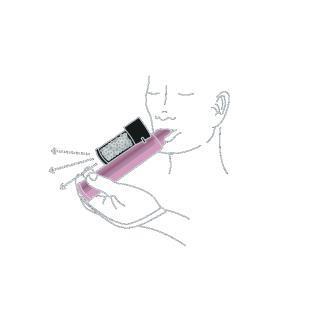
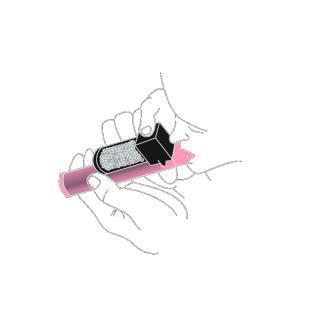
- 3. If stronger pain relief is needed, cover the hole located in the upper part of the transparent carbon chamber with your finger. The medical staff will indicate the location of this hole.
- 4. It is not necessary to breathe through the inhaler all the time. Medical staff will encourage you to take breaks in inhalation, as this will allow for a longer duration of pain relief.

Medical staff will encourage you to take breaks in inhalation, as this will allow for a longer duration of pain relief.
- 5. Use the inhalations for as long as the medical staff recommends or until the maximum recommended dose of the medicine is reached.
Do not give the medicine to another person, even if their symptoms are the same.
Overdose of Penthrox
The patient uses the medicine under the supervision of medical staff trained in its administration, so the likelihood of overdose is extremely low. A maximum of two bottles of the medicine can be used at one time. Exceeding the maximum dose of Penthrox may result in kidney damage. If you suspect that you have taken a higher dose than recommended, inform the medical staff immediately. In case of questions or doubts, consult your doctor or pharmacist.
4. Side effects
Like all medicines, Penthrox can cause side effects, although not everybody gets them.
Severe side effects
If you experience any of the following symptoms, tell your doctor or pharmacist immediately:
- Severe allergic reactions, including symptoms such as breathing difficulties and/or face swelling.
- Liver symptoms, such as loss of appetite, nausea, vomiting, jaundice (yellowing of the skin and/or eyes), dark urine, pale stools, pain or tenderness in the right side (below the ribcage).
- Kidney symptoms, such as decreased or increased urination, swelling of the feet or lower legs.
- Slow and shallow breathing or other breathing difficulties (symptoms of respiratory depression) (frequency cannot be estimated from the available data).
The above side effects can be life-threatening, so inform your doctor or pharmacist immediately if they occur.
Other side effects
Very common(may affect more than 1 in 10 people)
- Dizziness
Common side effects(may affect up to 1 in 10 people):
- Drowsiness
- Headache or nausea
- Euphoria
- Feeling of intoxication
- Taste disturbances
- Cough
Uncommon side effects(may affect up to 1 in 100 people):
- Anxiety or depression
- Attention disturbances
- Inappropriate emotions or behaviors
- Repetition of words or difficulties in speaking
- Memory loss
- Numbness or tingling in hands and feet
- Numbness
- Visual disturbances
- Redness of the skin
- Low or high blood pressure
- Dry mouth
- Discomfort or itching in the mouth
- Increased saliva production
- Increased appetite
- Vomiting
- Sweating
- Fatigue
- Malaise
- Chills
- Feeling of relaxation
Frequency not known(frequency cannot be estimated from the available data):
- Hypersensitivity
- Mood disturbances
- Nervousness or agitation
- Feeling of detachment from reality
- Disorientation
- Altered states of consciousness
- Choking
- Shallow breathing
- Uncontrolled eye movements
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to: Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported directly to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Penthrox
Keep the medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the packaging after EXP. The expiry date refers to the last day of the month. There are no special precautions for the storage of this medicinal product. The doctor or medical staff will dispose of any remaining Penthrox and the Penthrox inhaler in accordance with local regulations.
6. Contents of the pack and other information
What Penthrox contains
- The active substance of Penthrox is methoxyflurane. One sealed bottle contains 3 ml of methoxyflurane 99.9%.
- The other ingredient is: butylhydroxytoluene (E 321).
What Penthrox looks like and contents of the pack
Penthrox is a clear, almost colorless, volatile liquid with a characteristic fruity smell. The medicinal product Penthrox is available in the following packs: 10 bottles of 3 ml, in a cardboard box. 1 or 10 sets. The set includes: one bottle of 3 ml methoxyflurane 99.9%, one Penthrox inhaler, and one carbon chamber with a security lock. The bottle has a capacity of 5 ml, made of orange glass type I, with a PET insert, a POM (polyoxymethylene) screw cap, and a tamper-evident seal. Not all pack sizes may be marketed.
Marketing authorization holder
Medical Developments NED B.V. Strawinskylaan 411, WTC Tower A, 1077 XX Amsterdam, Netherlands
Manufacturer
MIAS Pharma Limited, Suite 1, First Floor, Stafford House, Strand Road, Portmarnock, Co. Dublin, Ireland
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria: Penthrop, Belgium: Methoxyflurane Medical Developments NED, Croatia: Penthrox, Czech Republic: Penthrox, Denmark: Penthrox, Finland: Penthrox, France: Methoxyflurane Medical Developments NED, Spain: Penthrox, Iceland: Penthrox, Ireland: Methoxyflurane Medical Developments MD&P, Luxembourg: Methoxyflurane Medical Developments NED, Germany: Penthrox, Norway: Penthrox, Poland: Penthrox, Portugal: Penthrox, Slovakia: Penthrox, Slovenia: Penthrox, Sweden: Penthrox, Italy: Penthrox. Date of last revision of the leaflet:05/2023
Information for medical staff
The following information is intended for medical staff only: The instructions below describe the proper preparation of the Penthrox inhaler for use and the correct way to use it.
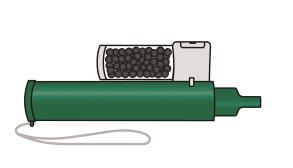
- 1. Make sure the carbon chamber is placed in the slot at the top of the Penthrox inhaler.
- 2. Remove the bottle cap manually. Alternatively: use the base of the Penthrox inhaler to loosen the bottle cap – insert it into the base and turn it 180° (half a turn). Separate the inhaler from the bottle and remove the cap manually.
- 3. Hold the Penthrox inhaler at a 45° angle and pour the entire contents of one bottle of Penthrox into its base, while rotating the inhaler several times around its axis.

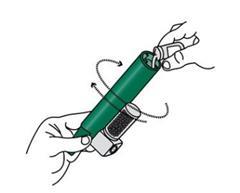
- 4. Put the security strap on the patient's wrist. The patient breathes through the Penthrox inhaler mouthpiece to relieve pain. Instruct the patient to take the first breaths through the inhaler cautiously and then start breathing normally.

- 5. The patient also exhales into the Penthrox inhaler. The exhaled vapors pass through the carbon chamber to capture any remaining methoxyflurane.
- 6. If stronger pain relief is needed, the patient (during inhalation) can cover the hole located in the upper part of the carbon chamber with their finger.
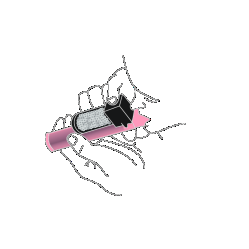
- 7. If further pain relief is needed after using the first bottle, a second bottle of the medicine can be used, if available. Alternatively, a bottle from a new pack can be used. Prepare for use in the same way as for the previous dose (steps 2 and 3).
There is no need to replace the carbon chamber. Place the used bottle in the included plastic bag.
- 8. Inform the patient that in order to achieve the desired pain relief effect, they should take breaks in inhalation. Continuous inhalation shortens the duration of pain relief. The patient should use the minimum dose that allows for effective pain control.
- 9. Close the empty medicine bottle. Place the used Penthrox inhaler and the empty bottle in a sealable plastic bag and dispose of them properly.
The doctor, nurse, paramedic, or person trained in the use of Penthrox must provide the patient with the patient information leaflet and familiarize them with its contents.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterMIAS Pharma Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PenthroxDosage form: Tablets, 30 mgActive substance: nefopamManufacturer: Holsten Pharma GmbH Laboratories BTTPrescription requiredDosage form: Tablets, 30 mgActive substance: nefopamManufacturer: Przedsiębiorstwo Farmaceutyczne Jelfa S.A.Prescription requiredDosage form: Aerosol, (27 mg + 25 mg)/mlActive substance: cannabinoidsPrescription required
Alternatives to Penthrox in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Penthrox in Ukraine
Alternative to Penthrox in Spain
Online doctors for Penthrox
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Penthrox – subject to medical assessment and local rules.














