How to use Sandostatin Lar
Leaflet accompanying the packaging: patient information
SANDOSTATIN LAR 10 mg powder and solvent for suspension for injection
SANDOSTATIN LAR 20 mg powder and solvent for suspension for injection
SANDOSTATIN LAR 30 mg powder and solvent for suspension for injection
Octreotide
You should carefully read the contents of this leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Sandostatin LAR and what is it used for
- 2. Important information before using Sandostatin LAR
- 3. How to use Sandostatin LAR
- 4. Possible side effects
- 5. How to store Sandostatin LAR
- 6. Contents of the packaging and other information
1. What is Sandostatin LAR and what is it used for
Sandostatin LAR is a synthetic derivative of somatostatin, a substance that occurs naturally in the human body, which inhibits the action of certain hormones, such as growth hormone. Sandostatin LAR acts stronger than somatostatin and its action lasts longer.
Sandostatin LAR is used
- in the treatment of acromegaly. Acromegaly is a disease in which the body produces too much growth hormone. In healthy individuals, growth hormone controls the growth of tissues, organs, and bones. Too much growth hormone causes an increase in the size of bones and tissues, especially the hands and feet. Sandostatin LAR significantly alleviates the symptoms of acromegaly, which include headache, excessive sweating, numbness of hands and feet, fatigue, and joint pain. In most cases, excessive production of growth hormone is due to the enlargement of the pituitary gland (pituitary adenoma); treatment with Sandostatin LAR may reduce the size of the adenoma.
Sandostatin LAR is used in the treatment of patients with acromegaly:
- in whom other types of treatment for acromegaly (surgical treatment or radiotherapy) are not indicated or are ineffective;
- after radiotherapy, during the transitional period, before the full effect of radiotherapy is achieved.
- to alleviate symptoms associated with excessive production of certain specific hormones and other substances by the stomach, intestines, and pancreas. Excessive production of hormones and other natural substances can be caused by certain rare diseases of the stomach, intestines, or pancreas. This disrupts the body's natural hormonal balance and is the cause of many symptoms such as facial flushing, diarrhea, low blood pressure, rash, and weight loss. Treatment with Sandostatin LAR helps control these symptoms.
- in the treatment of neuroendocrine tumors located in the intestine (e.g., in the appendix, small intestine, or colon)
Neuroendocrine tumors are rare tumors located in different parts of the body. Sandostatin LAR is also used to inhibit the growth of this type of tumor located in the intestine (e.g., in the appendix, small intestine, or colon).
- in the treatment of pituitary tumors that secrete too much thyroid-stimulating hormone (TSH). Too much TSH leads to hyperthyroidism. Sandostatin LAR is used in patients with pituitary tumors that secrete too much TSH:
- when other types of treatment (surgical treatment or radiotherapy) are not suitable or are ineffective;
- after radiotherapy, during the transitional period, before radiotherapy achieves its full effect.
2. Important information before using Sandostatin LAR
You should follow all the doctor's recommendations. They may differ from the information contained in this leaflet.
You should read the following information before using Sandostatin LAR.
When not to use Sandostatin LAR:
- if the patient is allergic to octreotide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Sandostatin LAR, you should discuss with your doctor:
- if you know that you have or have had gallstones in the past or if you have any symptoms such as fever, chills, abdominal pain, or yellowing of the skin and eyes; you should tell your doctor, as long-term use of Sandostatin LAR may cause gallstones. Your doctor may order periodic gallbladder checks.
- If you have diabetes, as Sandostatin LAR may affect blood sugar levels. If you have diabetes, your blood sugar levels should be regularly monitored.
- If you have had a reduced level of vitamin B12 in the past, your doctor may order periodic checks of vitamin B12 levels.
Examinations and check-ups
If you are being treated with Sandostatin LAR for a long time, your doctor may order periodic checks of thyroid function.
Your doctor will monitor your liver function.
Your doctor may order a check of pancreatic enzyme activity.
Children
There is limited experience with the use of Sandostatin LAR in children.
Sandostatin LAR and other medicines
You should tell your doctor or pharmacist about all the medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
During treatment with Sandostatin LAR, you can usually continue taking other medicines.
However, it has been reported that Sandostatin LAR affects the action of certain medicines, such as cimetidine, cyclosporine, bromocriptine, quinidine, and terfenadine.
If you are taking a medicine to control blood pressure (e.g., a beta-adrenergic receptor antagonist or a calcium channel blocker), your doctor may adjust the dosage.
In patients with diabetes, it may be necessary to adjust the insulin dose.If you are to receive treatment with lutetium (Lu) oxodotreotide, a radiopharmaceutical therapy, your doctor may interrupt and/or adjust treatment with Sandostatin LAR for a short period.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor before using this medicine.
Sandostatin LAR may only be used during pregnancy if clearly necessary.
Women of childbearing age should use effective methods of contraception during treatment.
You should not breastfeed while using Sandostatin LAR. It is not known whether Sandostatin LAR passes into breast milk.
Driving and using machines
Sandostatin LAR has no or negligible influence on the ability to drive and use machines. However, certain side effects that may occur during treatment with Sandostatin LAR, such as headache and fatigue, may reduce the patient's ability to drive and use machines safely.
Sandostatin LAR contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per vial, which means that the medicine is considered "sodium-free".
3. How to use Sandostatin LAR
Sandostatin LAR should only be administered by deep intramuscular injection into the buttock.
During long-term administration, injections should be performed alternately in the left and right buttock.
Using a higher dose of Sandostatin LAR than recommended:
After an overdose of Sandostatin LAR, no life-threatening reactions have been reported.
Symptoms of overdose include: hot flashes, frequent urination, fatigue, depression, anxiety, and lack of concentration.
If you feel that you have taken too much of this medicine and you experience the above symptoms, you should immediately tell your doctor.
Missing a dose of Sandostatin LAR:
If an injection is not administered at the right time, it should be administered as soon as possible, and then treatment should be continued as before. Administering a dose a few days later is not harmful, but it may cause a temporary recurrence of disease symptoms until the planned treatment schedule is resumed.
Stopping treatment with Sandostatin LAR:
After stopping treatment with Sandostatin LAR, disease symptoms may recur. Therefore, you should not stop using Sandostatin LAR without consulting your doctor.
If you have any further doubts about using this medicine, you should consult your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects can be serious. You should immediately inform your doctor if you experience any of the following symptoms:
Very common(may affect more than 1 in 10 people):
- Gallstones causing sudden back pain.
- High blood sugar levels.
Common(may affect up to 1 in 10 people):
- Hypothyroidism (underactive thyroid) affecting heart rate, appetite, or weight; fatigue, feeling cold, or swelling of the front of the neck.
- Changes in thyroid function test results.
- Cholecystitis; symptoms may include pain in the right upper abdomen, fever, nausea, yellowing of the skin and eyes (jaundice).
- Low blood sugar levels.
- Impaired glucose tolerance.
- Slow heart rate.
Uncommon(may affect up to 1 in 100 people):
- Thirst, low urine output, dark urine, dry, reddened skin.
- Fast heart rate.
Other serious side effects
- Hypersensitivity reactions (allergy), including skin rash.
- A type of allergic reaction (anaphylaxis), which can cause difficulty swallowing or breathing, swelling, and tingling, with possible low blood pressure, dizziness, or loss of consciousness.
- Pancreatitis; symptoms may include sudden pain in the upper abdomen, nausea, vomiting, diarrhea.
- Hepatitis; symptoms may include yellowing of the skin and eyes (jaundice), nausea, vomiting, loss of appetite, general malaise, itching, pale urine.
- Irregular heart rate.
- Low platelet count; this may cause increased bleeding or bruising.
If you notice any of the above symptoms, you should immediately tell your doctor.
Other side effects:
If you notice any of the following side effects, you should tell your doctor, pharmacist, or nurse. These effects are usually mild and tend to disappear as treatment progresses.
Very common(may affect more than 1 in 10 people):
- Diarrhea.
- Abdominal pain.
- Nausea.
- Constipation.
- Bloating with gas.
- Headache.
- Pain at the injection site.
Common(may affect up to 1 in 10 people):
- Discomfort in the stomach after eating (indigestion).
- Vomiting.
- Feeling of fullness in the stomach.
- Fatty stools.
- Loose stools.
- Discoloration of stools.
- Dizziness.
- Loss of appetite.
- Changes in liver function test results.
- Hair loss.
- Shortness of breath.
- Weakness.
If you experience any side effects, you should tell your doctor, nurse, or pharmacist.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C
02-222 Warsaw
tel.: 22 49-21-301
fax: 22 49-21-309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Sandostatin LAR
The medicine should be stored out of sight and reach of children.
Store in the original packaging to protect from light.
Store in the refrigerator (2°C – 8°C). Do not freeze.
On the day of injection, Sandostatin LAR can be stored at a temperature below 25°C.
Do not store the prepared Sandostatin LAR (the reconstituted suspension must be used immediately).
Do not use this medicine after the expiry date stated on the label and packaging after "EXP" and "Expiry date (EXP)". The expiry date refers to the last day of the month stated.
Do not use this medicine if you notice any foreign particles or a change in color.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Sandostatin LAR contains
- The active substance of the medicine is octreotide. One vial contains 10 mg, 20 mg, or 30 mg of octreotide (as octreotide acetate).
- The other ingredients are: in the powder (vial): poly(DL-lactide-co-glycolide), mannitol (E 421). in the solvent (ampoule-syringe): carmellose sodium, mannitol (E 421), poloxamer 188, water for injections.
What Sandostatin LAR looks like and contents of the pack
A single pack contains one glass vial 6 ml closed with a bromobutyl rubber stopper and an aluminum cap with a tear-off seal, containing the powder for suspension for injection, and one glass ampoule-syringe 3 ml, protected by two chlorobutyl rubber stoppers (at the front and at the plunger), containing 2 ml of solvent for suspension for injection, packaged together in a sealed blister pack with a vial connector and one safety needle for injection.
Marketing authorization holder
Novartis Poland Sp. z o.o.
Marynarska 15
02-674 Warsaw
Tel. + 48 22 375 48 88
Manufacturer/Importer
Novartis Poland Sp. z o.o.
Marynarska 15
02-674 Warsaw
Novartis Pharma GmbH
Jakov-Lind-Straße 5, Top 3.05
1020 Vienna
Austria
Novartis Pharma NV
Medialaan 40 Bus 1
Vilvoorde, B-1800
Belgium
Novartis Healthcare A/S
Edvard Thomsens Vej 14
Copenhagen S, 2300
Denmark
Novartis Finland Oy
Metsäneidonkuja 10
Espoo, FI-02130
Finland
Novartis Pharma SAS
8-10 rue Henri Sainte-Claire Deville
92500 Rueil Malmaison
France
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nuremberg
Germany
Novartis (HELLAS) SA
12th km National Road Athinon-Lamias
Metamorfosi Attiki, 14451
Greece
Novartis Hungáry Kft.
Vasút u.13.
Budaörs, 2040
Hungary
Novartis Farma S.p.A.
Via Provinciale Schito 131
80058 Torre Annunziata (NA)
Italy
Novartis Farma S.p.A.
Viale Luigi Sturzo 43
- 20154 – Milan (MI) Italy
Novartis Farma - Produtos Farmacêuticos S.A.
Avenida Professor Doutor Cavaco Silva, n.10E, Taguspark
Porto Salvo, 2740-255
Portugal
Novartis Farmacéutica S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Sverige AB
Torshamnsgatan 48
164 40 Kista
Sweden
Novartis Pharma B.V.
Haaksbergweg 16
1101 BX Amsterdam
Netherlands
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Sandostatin LAR
Austria, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Malta, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden
Sandostatine LAR
Belgium, Luxembourg, Netherlands
Sandostatina LAR
Italy, Portugal
Sandostatine L.P.
France
Date of last revision of the leaflet: 10/2024
Information intended only for healthcare professionals:
What dose of Sandostatin LAR should be used
Acromegaly
It is recommended to start treatment with Sandostatin LAR at a dose of 20 mg every 4 weeks for 3 months. Patients receiving Sandostatin subcutaneously may start treatment with Sandostatin LAR the day after the last subcutaneous dose of Sandostatin. Then, the dose should be adjusted based on the level of growth hormone (GH) and insulin-like growth factor 1/somatomedin C (IGF-1) in the blood and clinical symptoms.
For patients in whom after 3 months the clinical symptoms and biochemical parameters (GH; IGF-1) are not fully controlled (GH levels are still higher than 2.5 micrograms/l), the dose of Sandostatin LAR may be increased to 30 mg every 4 weeks. If after another 3 months the GH, IGF-1, and/or other symptoms are still not satisfactorily controlled during treatment with a dose of 30 mg, the dose of Sandostatin LAR may be increased to 40 mg every 4 weeks.
For patients in whom the level of GH is consistently below 1 microgram/l, and the level of IGF-1 in the blood has normalized, and the earliest disappearing objective and subjective symptoms of acromegaly have regressed after 3 months of treatment with a dose of 20 mg, Sandostatin LAR may be used at a dose of 10 mg every 4 weeks. However, especially in this group of patients, it is recommended to closely monitor the effectiveness of treatment by measuring GH and IGF-1 levels in the blood and assessing clinical objective and subjective symptoms during treatment with this low dose of Sandostatin LAR.
For patients receiving a fixed dose of Sandostatin LAR, GH and IGF-1 levels should be determined every 6 months.
Hormonally active tumors of the stomach, intestines, and pancreas
- Treatment of patients with symptoms associated with hormonally active neuroendocrine tumors of the stomach, intestines, and pancreasIt is recommended to start treatment with Sandostatin LAR at a dose of 20 mg every 4 weeks. Patients receiving Sandostatin subcutaneously should continue this treatment at the previously effective dose for 2 weeks after the first injection of Sandostatin LAR.
For patients in whom after 3 months of treatment there is a satisfactory alleviation of symptoms and improvement in biological markers, the dose of Sandostatin LAR may be reduced to 10 mg every 4 weeks.
For patients in whom after 3 months of treatment there is only partial alleviation of symptoms, the dose of Sandostatin LAR may be increased to 30 mg every 4 weeks.
On days when, despite treatment with Sandostatin LAR, symptoms associated with tumors of the stomach, intestines, and pancreas are exacerbated, it is recommended to administer an additional subcutaneous dose of Sandostatin at the dose used before the introduction of Sandostatin LAR. This may occur especially during the first 2 months of treatment, before the therapeutic level of octreotide is achieved.
- Treatment of patients with advanced neuroendocrine tumors originating from the midgut or with an unknown primary site, in whom the primary site is not located in the midgutThe recommended dose of Sandostatin LAR is 30 mg, administered every 4 weeks. Treatment with Sandostatin LAR to inhibit tumor growth should be continued in the absence of tumor progression.
Treatment of TSH-secreting adenomas
Treatment with Sandostatin LAR should be started at a dose of 20 mg every 4 weeks and continued for 3 months before possible dose adjustment. Then, the dose may be adjusted based on TSH and thyroid hormone secretion.
Instructions for preparing and administering Sandostatin LAR by deep intramuscular injection
ONLY FOR DEEP INTRAMUSCULAR INJECTION
Kit:
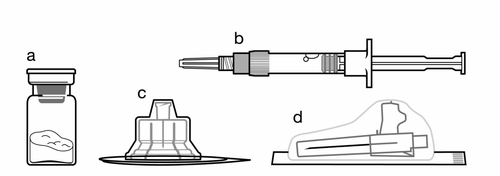
a
One vial containing Sandostatin LAR powder
b
One ampoule-syringe containing solvent for suspension for injection
c
One vial connector for reconstituting the medicinal product
d
One safety needle for injection
You should follow the instructions below to properly reconstitute Sandostatin LAR before administering a deep intramuscular injection.
For proper reconstitution of Sandostatin LAR, 3 points are critical. Failure to follow these may result in improper administration of the medicine.
Failure to meet the requirements presented may result in improper administration of the medicine.
- The injection kit must reach room temperature.You should remove the injection kit from the refrigerator and, before reconstituting the medicinal product, leave it at room temperature for at least 30 minutes, but no longer than 24 hours.
- After adding the solvent, you should leave the vial for 5 minutes to ensure that the powder is fully saturated with the solvent.
- After saturation, you should shake the vial vigorouslyin a horizontal direction for at least 30 seconds to form a uniform suspension.The suspension of Sandostatin LAR must be prepared immediatelybefore administration.
Sandostatin LAR may only be administered by trained medical personnel.
Step 1
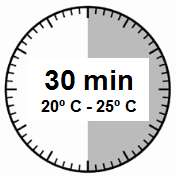
- Remove the Sandostatin LAR injection kit from the refrigerator where it was stored.
IMPORTANT: It is crucial to start the reconstitution process only when the injection kit has reached room temperature. You should leave the
injection kit at room temperature for at least
30 minutes before reconstitution, but no longer than 24 hours.
Note: The injection kit can be returned to the refrigerator if needed.
Step 2
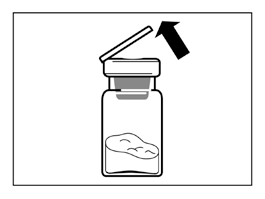
- Remove the plastic cap from the vial and wipe the rubber stopper of the vial with an alcohol swab.
- Remove the protective covering from the vial connector packaging, but DO NOT remove the vial connector from the packaging.
- Holding the vial connector by the packaging, place the vial connector on the vial and push it down until it clicks into place.
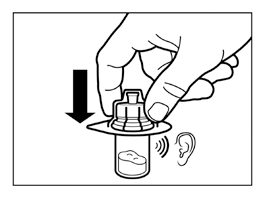
- Remove the packaging from the vial connector by lifting it straight up.
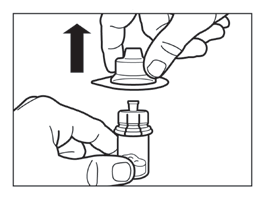
Step 3
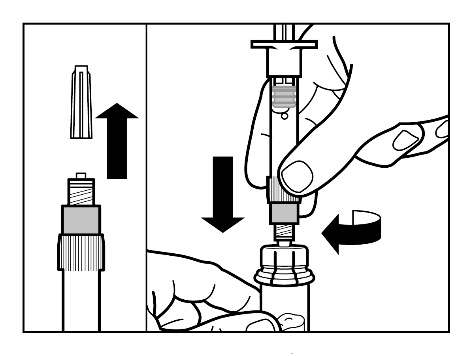
- Remove the cap from the ampoule-syringe containing the solvent and screw the syringe onto the vial connector.
- Slowly push the plunger down to the end, so that the entire solvent solution is transferred to the vial.
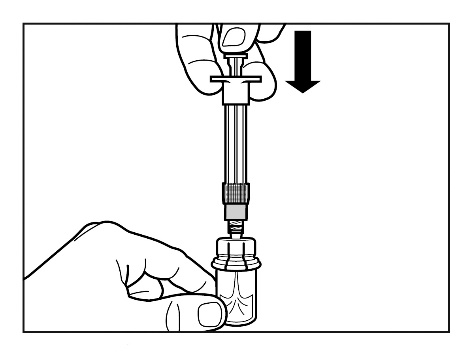
Step 4
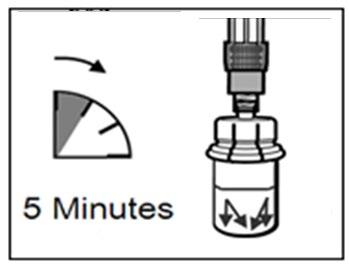
IMPORTANT: It is crucial to leave the vial for
5 minutes, to ensure that the powder is fully saturated with the solvent.
Note: If the plunger is pushed back up, this is a normal situation, caused by a slight overpressure in the vial.
5 minutes
- At this stage, you should prepare the patient for the injection.
Step 5
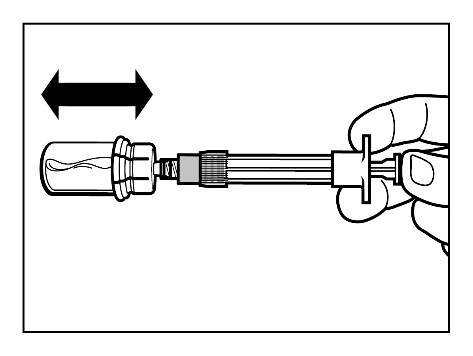
- After the powder is fully saturated with the solvent, you should check that the plunger is pushed down to the end of the syringe. IMPORTANT:Holding the plunger pushed down to the end firmlyshake the vial gentlyin a horizontal direction for at least30 seconds to form a uniform suspension.If some of the powder does not dissolve, repeat the gentle shaking for another 30 seconds.
Step 6
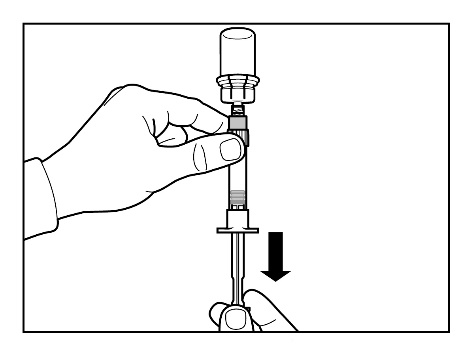
- Prepare the injection site by wiping it with an alcohol swab.
- Turn the syringe with the vial upside down and slowly pull the plunger back to draw the entire contents of the vial into the syringe.
- Unscrew the syringe from the vial connector.
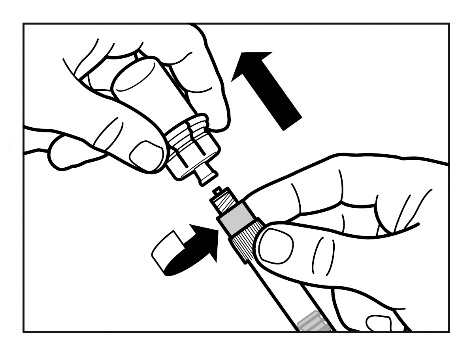
Step 7
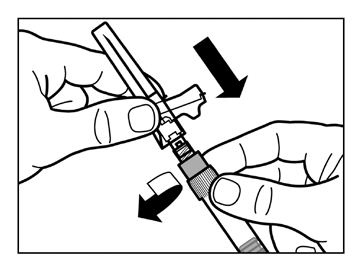
- Screw the safety needle for injection onto the syringe.
- Gently shake the syringe again to obtain a milky uniform suspension just before administration.
- Remove the protective cap from the needle by pulling it straight up.
- Gently tap the syringe with your fingers to make any visible air bubbles rise, and then expel them from the syringe. You shouldcheck that the injection site has not been contaminated.
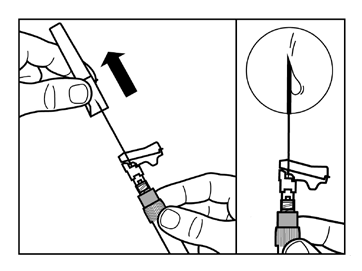
- The reconstituted Sandostatin LAR is now ready for immediateadministration - proceed to Step 8. Any delay may cause the sediment to settle.
Step 8
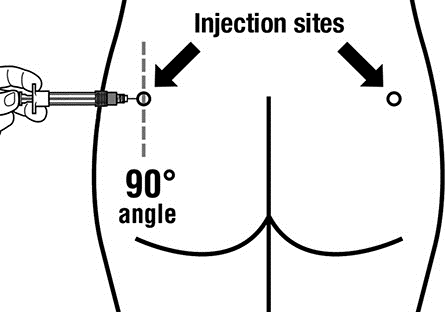
- Sandostatin LAR must be administered by deep intramuscular injection, NEVERintravenously.
- Insert the entire needle into the left or right buttock at a 90° angle to the skin surface.
- Slowly pull the plunger back to ensure that the needle is not in a blood vessel (change the position of the needle if it is in a blood vessel).
- Push the plunger with steady force until the syringe is empty. Remove the needle from the injection site and activate the needle guard (according to the instructions in Step 9).
Step 9
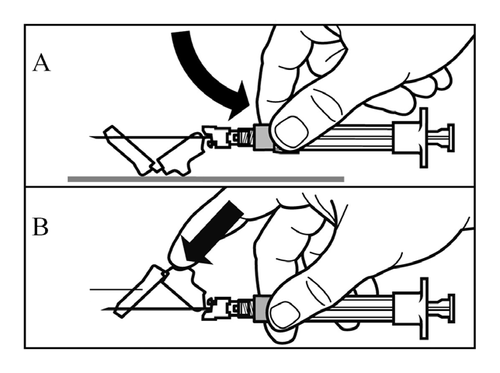
- Activate the needle guard by using one of the following 2 methods:
- press the folding part of the guard against a hard surface (Figure A)
- or press the folding part of the guard with your finger (Figure B).
- Proper activation will be confirmed by a loud click.
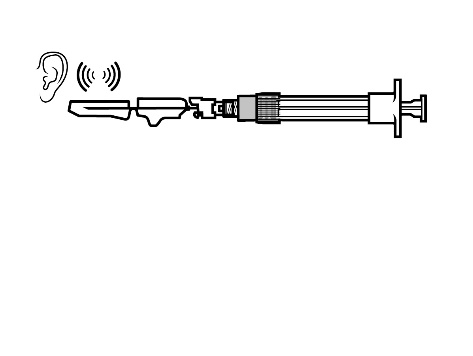
- Discard the syringe immediately (into an appropriate sharps disposal container).
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterNovartis Farma - Produtos Farmacêuticos, S.A. Novartis Farma S.p.A. Novartis Farma S.p.A. Novartis Farmacéutica, S.A. Novartis Finland Oy Novartis Healthcare A/S Novartis (Hellas) SA Novartis Hungary Kft. Novartis Pharma B.V. Novartis Pharma GmbH Novartis Pharma GmbH Novartis Pharma GmbH Novartis Pharma nv/sa Novartis Pharma SAS Novartis Poland Sp. z o.o. Novartis Sverige AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sandostatin LarDosage form: Powder, 10 mgActive substance: octreotideManufacturer: Merckle GmbH Pharmathen International S.A. PLIVA Hrvatska d.o.o. (PLIVA Croatia Ltd.) Teva Pharmaceuticals Europe B.V.Prescription requiredDosage form: Powder, 20 mgActive substance: octreotideManufacturer: Merckle GmbH Pharmathen International S.A. PLIVA Hrvatska d.o.o. (PLIVA Croatia Ltd.) Teva Pharmaceuticals Europe B.V.Prescription requiredDosage form: Powder, 30 mgActive substance: octreotideManufacturer: Merckle GmbH Pharmathen International S.A. PLIVA Hrvatska d.o.o. (PLIVA Croatia Ltd.) Teva Pharmaceuticals Europe B.V.Prescription required
Alternatives to Sandostatin Lar in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sandostatin Lar in Spain
Alternative to Sandostatin Lar in Ukraine
Online doctors for Sandostatin Lar
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sandostatin Lar – subject to medical assessment and local rules.