
How to use Sandostatin Lar
Leaflet accompanying the packaging: patient information
SANDOSTATIN LAR 10 mg powder and solvent for suspension for injection
SANDOSTATIN LAR 20 mg powder and solvent for suspension for injection
SANDOSTATIN LAR 30 mg powder and solvent for suspension for injection
Octreotide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Sandostatin LAR and what is it used for
- 2. Important information before using Sandostatin LAR
- 3. How to use Sandostatin LAR
- 4. Possible side effects
- 5. How to store Sandostatin LAR
- 6. Contents of the packaging and other information
1. What is Sandostatin LAR and what is it used for
Sandostatin LAR is a synthetic derivative of somatostatin, a substance that occurs naturally in the human body, which inhibits the action of certain hormones, such as growth hormone. Sandostatin LAR acts stronger than somatostatin and its action lasts longer.
Sandostatin LAR is used
- in the treatment of acromegaly. Acromegaly is a disease in which the body produces too much growth hormone. In healthy individuals, growth hormone controls the growth of tissues, organs, and bones. Too much growth hormone causes an increase in the size of bones and tissues, especially the hands and feet. Sandostatin LAR significantly alleviates the symptoms of acromegaly, which include headache, excessive sweating, numbness of the hands and feet, fatigue, and joint pain. In most cases, excessive production of growth hormone is due to an enlarged pituitary gland (pituitary adenoma); treatment with Sandostatin LAR may reduce the size of the adenoma.
Sandostatin LAR is used in the treatment of patients with acromegaly:
- in whom other types of treatment for acromegaly (surgical treatment or radiotherapy) are not indicated or are ineffective;
- after radiotherapy, during the transitional period, before the full effect of radiotherapy is achieved.
- to alleviate symptoms associated with excessive production of certain specific hormones and other substances by the stomach, intestines, and pancreas. Excessive production of hormones and other natural substances can be caused by certain rare diseases of the stomach, intestines, or pancreas. This disrupts the body's natural hormonal balance and is the cause of many symptoms such as facial flushing, diarrhea, low blood pressure, rash, and weight loss. Treatment with Sandostatin LAR helps control these symptoms.
- in the treatment of neuroendocrine tumors located in the intestine (e.g., in the appendix, small intestine, or colon)
Neuroendocrine tumors are rare tumors located in different parts of the body. Sandostatin LAR is also used to inhibit the growth of this type of tumor located in the intestine (e.g., in the appendix, small intestine, or colon).
- in the treatment of pituitary tumors that secrete too much thyroid-stimulating hormone (TSH). Too much TSH leads to hyperthyroidism. Sandostatin LAR is used in patients with pituitary tumors that secrete too much TSH:
- when other types of treatment (surgical treatment or radiotherapy) are not appropriate or are ineffective;
- after radiotherapy, during the transitional period, before radiotherapy achieves its full effect.
2. Important information before using Sandostatin LAR
Follow the doctor's instructions carefully. They may differ from the information contained in this leaflet.
When not to use Sandostatin LAR:
- if the patient is allergic to octreotide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Sandostatin LAR, discuss the following with your doctor:
- if the patient knows they have or have had gallstones in the past or if the patient experiences any symptoms such as fever, chills, abdominal pain, or yellowing of the skin and eyes; they should tell their doctor, as long-term use of Sandostatin LAR may cause gallstones. The doctor may order periodic gallbladder checks.
- If the patient has diabetes, as Sandostatin LAR may affect blood sugar levels. If the patient has diabetes, their blood sugar levels should be regularly monitored.
- If the patient has had a decreased level of vitamin B12 in the past, the doctor may order periodic checks of vitamin B12 levels.
Examinations and check-ups
If the patient is being treated with Sandostatin LAR for a long time, the doctor may order periodic thyroid function checks.
Children
There is limited experience with the use of Sandostatin LAR in children.
Sandostatin LAR and other medicines
Tell the doctor or pharmacist about all medicines the patient is currently taking or has recently taken, as well as any medicines the patient plans to take.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
Driving and using machines
Sandostatin LAR has no or negligible influence on the ability to drive and use machines. However, certain side effects that may occur during treatment with Sandostatin LAR, such as headache and fatigue, may reduce the patient's ability to drive and use machines safely.
Sandostatin LAR contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per vial, which means it is essentially 'sodium-free'.
3. How to use Sandostatin LAR
Sandostatin LAR should only be administered by deep intramuscular injection into the buttock.
Using a higher dose of Sandostatin LAR than recommended:
In case of overdose, no life-threatening reactions have been reported. Symptoms of overdose include: hot flashes, frequent urination, fatigue, depression, anxiety, and lack of concentration.
Missing a dose of Sandostatin LAR:
If an injection is missed, it should be administered as soon as possible, and then the treatment should be continued as before. Administering a dose a few days later is not harmful, but it may cause a temporary recurrence of disease symptoms until the planned treatment schedule is resumed.
Stopping treatment with Sandostatin LAR:
After stopping treatment with Sandostatin LAR, disease symptoms may recur. Therefore, treatment with Sandostatin LAR should not be stopped without consulting a doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects can be serious. The doctor should be informed immediately if the patient experiences any of the following symptoms:
Very common(may affect more than 1 in 10 people):
- Gallstones causing sudden back pain.
- High blood sugar levels.
Common(may affect up to 1 in 10 people):
- Hypothyroidism (underactive thyroid) affecting heart rate, appetite, or weight changes; fatigue, feeling cold, or swelling of the front of the neck.
- Changes in thyroid function test results.
- Gallbladder inflammation; symptoms may include pain in the right upper abdomen, fever, nausea, yellowing of the skin and eyes (jaundice).
- Low blood sugar levels.
- Impaired glucose tolerance.
- Slow heart rate.
Uncommon(may affect up to 1 in 100 people):
- Thirst, low urine output, dark urine color, dry, reddened skin.
- Fast heart rate.
Other serious side effects
- Allergic reactions (hypersensitivity), including skin rash.
- A type of allergic reaction (anaphylaxis) that can cause difficulty swallowing or breathing, swelling, and tingling, with possible low blood pressure, dizziness, or loss of consciousness.
- Pancreatitis; symptoms may include sudden pain in the upper abdomen, nausea, vomiting, diarrhea.
- Hepatitis; symptoms may include yellowing of the skin and eyes (jaundice), nausea, vomiting, loss of appetite, general malaise, itching, light-colored urine.
- Irregular heartbeat.
- Low platelet count; this can cause increased bleeding or bruising.
If the patient experiences any of the above symptoms, they should inform their doctor immediately.
Other side effects:
If the patient experiences any of the following side effects, they should tell their doctor, pharmacist, or nurse. These effects are usually mild and tend to disappear as treatment progresses.
Very common(may affect more than 1 in 10 people):
- Diarrhea.
- Abdominal pain.
- Nausea.
- Constipation.
- Bloating with gas.
- Headache.
- Pain at the injection site.
Common(may affect up to 1 in 10 people):
- Discomfort in the stomach after eating (indigestion).
- Vomiting.
- Feeling of fullness in the stomach.
- Fatty stools.
- Loose stools.
- Discoloration of stools.
- Dizziness.
- Loss of appetite.
- Changes in liver function test results.
- Hair loss.
- Shortness of breath.
- Weakness.
If side effects occur, the patient should tell their doctor, nurse, or pharmacist.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the patient should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder.
5. How to store Sandostatin LAR
The medicine should be stored out of sight and reach of children.
What Sandostatin LAR contains
- The active substance is octreotide. One vial contains 10 mg, 20 mg, or 30 mg of octreotide (as octreotide acetate).
- The other ingredients are: in the powder (vial): poly(DL-lactic-co-glycolic acid), mannitol (E 421), in the solvent (ampoule syringe): carmellose sodium, mannitol (E 421), poloxamer 188, water for injections.
What Sandostatin LAR looks like and contents of the pack
A single pack contains one glass vial of 6 ml, closed with a bromobutyl rubber stopper and an aluminum cap with a tear-off seal, containing powder for suspension for injection, and one glass ampoule syringe of 3 ml, protected by two chlorobutyl rubber stoppers (at the front and at the plunger), containing 2 ml of solvent for suspension for injection, packaged together in a sealed blister pack with a vial connector and one safety needle for injection.
Marketing authorization holder
Novartis Poland Sp. z o.o., ul. Marynarska 15, 02-674 Warsaw, Tel. +48 22 375 48 88
Manufacturer/Importer
Novartis Poland Sp. z o.o., ul. Marynarska 15, 02-674 Warsaw, Novartis Pharma GmbH, Jakov-Lind-Straße 5, Top 3.05, 1020 Wien, Austria, Novartis Pharma NV, Medialaan 40 Bus 1, Vilvoorde, B-1800, Belgium, Novartis Healthcare A/S, Edvard Thomsens Vej 14, København S, 2300, Denmark, Novartis Finland Oy, Metsäneidonkuja 10, Espoo, FI-02130, Finland, Novartis Pharma SAS, 8-10 rue Henri Sainte-Claire Deville, 92500 Rueil Malmaison, France, Novartis Pharma GmbH, Roonstrasse 25, 90429 Nürnberg, Germany, Novartis Pharma GmbH, Sophie-Germain-Strasse 10, 90443 Nürnberg, Germany, Novartis (HELLAS) SA, 12th km National Road Athinon-Lamias, Metamorfosi Attiki, 14451, Greece, Novartis Hungáry Kft., Vasút u.13., Budaörs, 2040, Hungary, Novartis Farma S.p.A., Via Provinciale Schito 131, 80058 Torre Annunziata (NA), Italy, Novartis Farma S.p.A., Viale Luigi Sturzo 43, 20154 – Milan (MI) Italy, Novartis Farma - Produtos Farmacêuticos S.A., Avenida Professor Doutor Cavaco Silva, n.10E, Taguspark, Porto Salvo, 2740-255, Portugal, Novartis Farmacéutica S.A., Gran Via de les Corts Catalanes, 764, 08013 Barcelona, Spain, Novartis Sverige AB, Torshamnsgatan 48, 164 40 Kista, Sweden, Novartis Pharma B.V., Haaksbergweg 16, 1101 BX Amsterdam, Netherlands
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Sandostatin LAR, Austria, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Malta, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sandostatine LAR, Belgium, Luxembourg, Netherlands, Sandostatina LAR, Italy, Portugal, Sandostatine L.P., France
Date of last revision of the leaflet: 10/2024
Information intended for healthcare professionals only:
What dose of Sandostatin LAR should be used
Acromegaly
It is recommended to start treatment with Sandostatin LAR at a dose of 20 mg every 4 weeks for 3 months. Patients receiving Sandostatin subcutaneously may start treatment with Sandostatin LAR the day after the last subcutaneous dose of Sandostatin.
Then, the dose should be adjusted based on the level of growth hormone (GH) and insulin-like growth factor 1/somatomedin C (IGF-1) in the blood and clinical symptoms.
In patients who after 3 months of treatment have not fully controlled clinical symptoms and biochemical parameters (GH; IGF-1), the dose of Sandostatin LAR may be increased to 30 mg every 4 weeks.
If after another 3 months the parameters of GH, IGF-1, and/or other symptoms are still not satisfactorily controlled during treatment with a dose of 30 mg, the dose of Sandostatin LAR may be increased to 40 mg every 4 weeks.
In patients who have a stable level of GH below 1 microgram/l and a normalized level of IGF-1 in the blood, and in whom the earliest disappearing symptoms of acromegaly have regressed after 3 months of treatment with a dose of 20 mg, Sandostatin LAR may be used at a dose of 10 mg every 4 weeks.
However, especially in this group of patients, it is recommended to closely monitor the effectiveness of treatment by measuring GH and IGF-1 levels in the blood and assessing clinical symptoms during treatment with this low dose of Sandostatin LAR.
In patients receiving a fixed dose of Sandostatin LAR, GH and IGF-1 levels should be determined every 6 months.
Instructions for preparing and administering Sandostatin LAR
FOR DEEP INTRAMUSCULAR INJECTION ONLY
Kit:
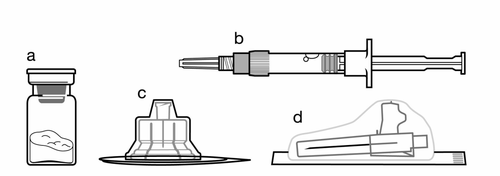
a
One vial containing Sandostatin LAR powder
b
One ampoule syringe containing solvent for suspension for injection
c
One vial connector for reconstituting the medicinal product
d
One safety needle for injection
Follow the instructions below to properly reconstitute Sandostatin LAR before administering the deep intramuscular injection.
Three key points are crucial for the proper reconstitution of Sandostatin LAR.
Failure to follow these instructions may result in improper administration of the medicine.
- The injection kit must reach room temperature.Remove the injection kit from the refrigerator and let it stand at room temperature for at least 30 minutes before reconstituting the medicinal product, but no longer than 24 hours.
- After adding the solvent, let the vial stand for 5 minutesto ensure that the powder is fully dissolved.
- After dissolution, shake the vial vigorouslyin a horizontal direction for at least 30 secondsto form a uniform suspension. The suspension of Sandostatin LAR must be prepared immediatelybefore administration.
Sandostatin LAR can only be administered by trained medical personnel.
Step 1
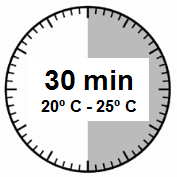
- Remove the Sandostatin LAR injection kit from the refrigerator where it was stored.
IMPORTANT:
It is crucial to start the reconstitution process only when the injection kit has reached room temperature. Let the kit stand at room temperature for at least 30 minutesbefore reconstituting, but no longer than 24 hours.
Note: The injection kit can be returned to the refrigerator if necessary.
Step 2
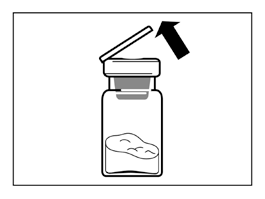
- Remove the plastic cap from the vial and wipe the rubber stopper of the vial with an alcohol swab.
- Remove the protective cover from the vial connector packaging, but do not remove the vial connector from the packaging.
- Holding the vial connector through the packaging, place the vial connector on the vial and push it down until it clicks into place.
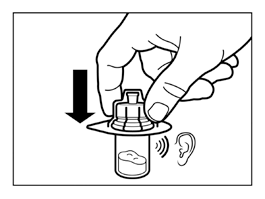
- Remove the packaging from the vial connector by lifting it straight up.
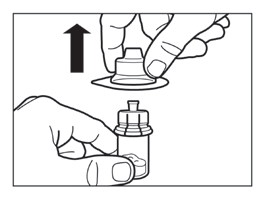
Step 3
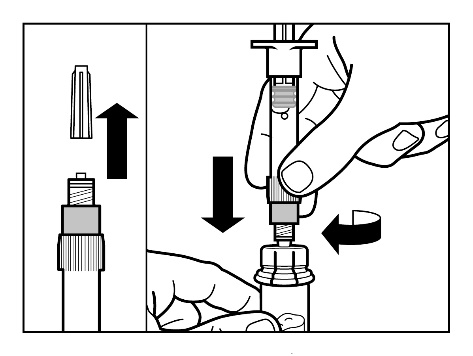
- Remove the cap from the ampoule syringe containing the solvent and screw the syringe to the vial connector.
- Slowly push the plunger all the way down to transfer all the solvent into the vial.
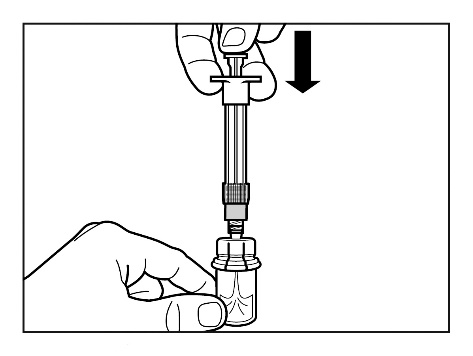
Step 4
IMPORTANT:
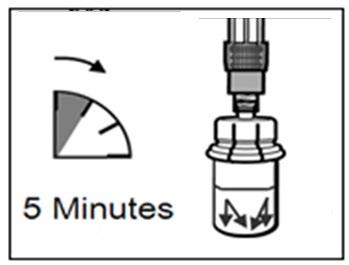
Let the vial stand for 5 minutesto ensure that the powder is fully dissolved.
Note: If the plunger is pushed back up, this is a normal situation due to slight overpressure in the vial.
5 minutes
- At this stage, prepare the patient for the injection.
Step 5
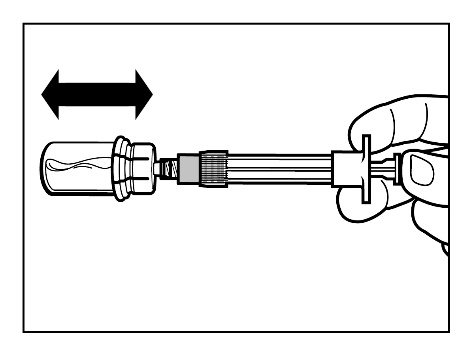
- After the powder has dissolved, check that the plunger is fully pushed down. IMPORTANT:Holding the plunger fully pushed down, gentlyshake the vial in a horizontal direction for at least30 seconds to form a uniform suspension (homogeneous milky suspension). If some powder has not dissolved, repeat the gentle shaking for another 30 seconds.
Step 6
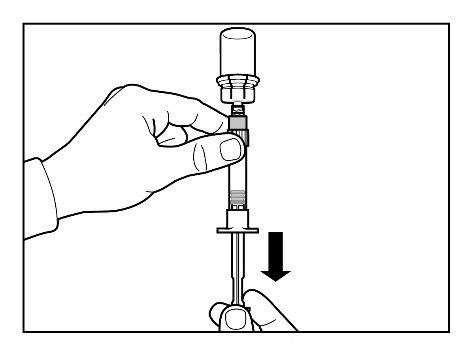
- Prepare the injection site by wiping it with an alcohol swab.
- Turn the syringe with the vial upside down and slowly pull the plunger to draw the entire contents of the vial into the syringe.
- Unscrew the syringe from the vial connector.
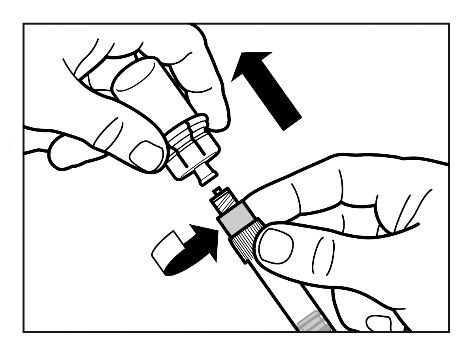
Step 7
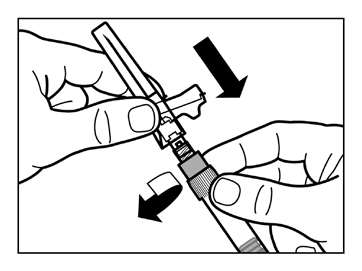
- Attach the safety needle to the syringe.
- Gently shake the syringe again to obtain a uniform milky suspension just before administration.
- Remove the protective cap from the needle with a straight upward motion.
- Gently tap the syringe with your fingers to move any visible air bubbles to the top, and then expel them from the syringe. Make surethe injection site has not been contaminated.
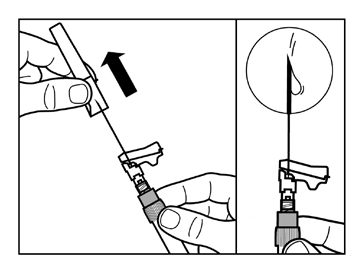
- The reconstituted Sandostatin LAR is now ready for immediateadministration - proceed to Step 8. Any delay may cause the sediment to settle.
Step 8
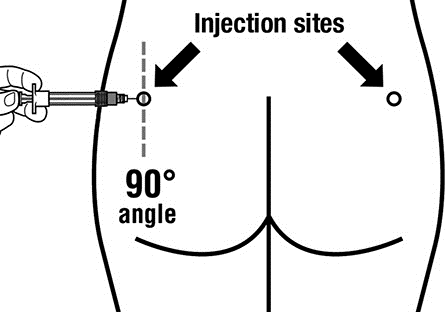
- Sandostatin LAR must be administered by deep intramuscular injection, NEVERintravenously.
- Insert the entire needle into the left or right buttock at a 90° angle to the skin surface.
- Slowly pull the plunger back to ensure that the needle is not in a blood vessel (change the needle position if it is in a blood vessel).
- Push the plunger with steady force until the syringe is empty. Remove the needle from the injection site and activate the needle guard (following the instructions in Step 9).
Step 9
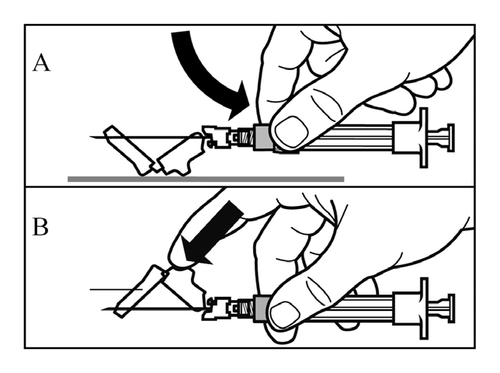
- Activate the needle guard using one of the following two methods:
- press the folding part of the guard against a hard surface (Figure A)
- or press the folding part of the guard with your finger (Figure B).
- Proper activation will be confirmed by a loud click.
- Discard the syringe immediately (in an appropriate sharps disposal container).
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterNovartis Farma - Produtos Farmacêuticos, S.A. Novartis Farma S.p.A. Novartis Farmacéutica, S.A. Novartis Finland Oy Novartis Healthcare A/S Novartis (Hellas) SA Novartis Hungary Kft. Novartis Pharma B.V. Novartis Pharma GmbH Novartis Pharma GmbH Novartis Pharma GmbH Novartis Pharma nv Novartis Pharma SAS Novartis Poland Sp. z o.o. Novartis Sverige AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sandostatin LarDosage form: Powder, 10 mgActive substance: octreotideManufacturer: Merckle GmbH Pharmathen International S.A. PLIVA Hrvatska d.o.o. (PLIVA Croatia Ltd.) Teva Pharmaceuticals Europe B.V.Prescription requiredDosage form: Powder, 20 mgActive substance: octreotideManufacturer: Merckle GmbH Pharmathen International S.A. PLIVA Hrvatska d.o.o. (PLIVA Croatia Ltd.) Teva Pharmaceuticals Europe B.V.Prescription requiredDosage form: Powder, 30 mgActive substance: octreotideManufacturer: Merckle GmbH Pharmathen International S.A. PLIVA Hrvatska d.o.o. (PLIVA Croatia Ltd.) Teva Pharmaceuticals Europe B.V.Prescription required
Alternatives to Sandostatin Lar in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sandostatin Lar in Espanha
Alternative to Sandostatin Lar in Ukraine
Online doctors for Sandostatin Lar
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sandostatin Lar – subject to medical assessment and local rules.







