
Rolprina Sr

Ask a doctor about a prescription for Rolprina Sr

How to use Rolprina Sr
Leaflet accompanying the packaging: patient information
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Rolpryna SR (Ropinirole Krka 8 mg)
8 mg, prolonged-release tablets
Ropinirol
Rolpryna SR and Ropinirole Krka 8 mg are different trade names for the same drug.
It is necessary to carefully read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
- The leaflet should be kept so that it can be re-read if necessary.
- In case of any doubts, the doctor or pharmacist should be consulted.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their disease are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Rolpryna SR and what is it used for
- 2. Important information before taking Rolpryna SR
- 3. How to take Rolpryna SR
- 4. Possible side effects
- 5. How to store Rolpryna SR
- 6. Contents of the packaging and other information
1. What is Rolpryna SR and what is it used for
The active substance of Rolpryna SR is ropinirole, which belongs to a group of medicines called dopamine agonists. Dopamine agonists work in the brain in a similar way to a naturally occurring substance called dopamine.
Rolpryna SR, prolonged-release tablets are used to treat
Parkinson's disease.
In patients with Parkinson's disease, there is a low concentration of dopamine in some parts of the brain.
Ropinirole works in a similar way to naturally occurring dopamine in the brain and thus helps to alleviate the symptoms of Parkinson's disease.
2. Important information before taking Rolpryna SR
When not to take Rolpryna SR
- if the patient is allergicto ropinirole or any of the other ingredients of this medicine (listed in section 6),
- if the patient has severe kidney disease,
- if the patient has liver disease.The doctor should be informed if any of the above situations apply to the patient.
Warnings and precautions
Before starting treatment with Rolpryna SR, the doctor or pharmacist should be consulted:
- if the patient is pregnantor thinks they may be pregnant,
- if the patient is breastfeeding,
- if the patient is under 18 years old, Page 1 7
- if the patient has severe heart disease,
- if the patient has severe mental disorders,
- if the patient has particular tendencies and (or) behaviors(see section 4),
- if the patient has intolerance to sugars (such as lactose). The doctor should be informed if any of the above situations apply to the patient. The doctor may decide that Rolpryna SR is not a suitable medicine for the patient or may recommend additional monitoring during treatment.
The doctor should be told if the patient or their family/caregiver notices that the patient starts to feel the urge or desire to behave in an unusual way and if the patient cannot resist the impulse, drive, or temptation to engage in activities that may harm them or others. These phenomena are called impulse control disorders and may manifest as behaviors such as compulsive gambling, excessive appetite, or the need to spend money, excessive sexual drive, or increased frequency and intensity of thoughts or feelings about sex. The doctor may consider it appropriate to change the dose or discontinue the medicine.
The doctor should be informed if symptoms such as depression, apathy, anxiety, fatigue, excessive sweating, or pain occur after stopping treatment or reducing the dose of ropinirole [called dopamine agonist withdrawal syndrome (DAWS)]. If these symptoms persist for more than a few weeks, the doctor may modify the treatment.
The doctor should be informed if the patient, a family member, or caregiver notices that the patient is developing episodes of overactivity, euphoria, or irritability (mania symptoms). These may occur with or without impulse control disorders (see above). The doctor may consider it necessary to adjust the dose or discontinue the medicine.
During treatment with Rolpryna SR
The doctor should be contacted if the patient or their family member observes the occurrence of any unusual behavior in the patient (such as an uncontrollable urge to gamble or increased sexual drive) during treatment with Rolpryna SR. The doctor may recommend adjusting the dose or discontinuing the medicine.
Smoking and taking Rolpryna SR
The doctor should be toldabout starting or stopping smoking while taking Rolpryna SR. The doctor may decide to adjust the dose.
Rolpryna SR and other medicines
The doctor or pharmacist should be told about all medicines the patient is currently taking or has recently taken, including herbal products and other medicines available without a prescription.
It should be remembered to tell the doctor or pharmacist if the patient starts taking any other medicine while taking Rolpryna SR.
Some medicines may affect the action of Rolpryna SR or increase the risk of side effects. Rolpryna SR may also affect the action of other medicines.
These medicines include:
- fluvoxamine (an antidepressant),
- medicines used to treat other mental disorders, such as sulpiride,
- hormone replacement therapy (HRT),
- metoclopramide, which is used to treat nausea and heartburn,
- antibiotics: ciprofloxacin or enoxacin,
- any other medicine used to treat Parkinson's disease. The doctor should be informed if the patient is taking or has recently taken any of these medicines.
Page 2 7
Additional blood tests will be necessaryif the patient is taking Rolpryna SR at the same time as:
- vitamin K antagonists (used to thin the blood), such as warfarin.
Taking Rolpryna SR with food and drink
Rolpryna SR can be taken with or without food.
Pregnancy and breastfeeding
Rolpryna SR is not recommended during pregnancy unless the doctor decides that the benefits of taking Rolpryna SR outweigh the risks to the unborn child. Rolpryna SR is not recommended during breastfeeding, as it may affect milk production.
The doctor should be informed immediately if the patient is pregnant, thinks they may be pregnant, or plans to become pregnant. The doctor will also advise if the patient is breastfeeding or plans to breastfeed. The doctor may recommend discontinuing Rolpryna SR.
Driving and using machines
Rolpryna SR may cause drowsiness. Uncontrollable drowsinessmay occur, as well as sudden and unexpected sleep attacks without warning.
Ropinirole may cause hallucinations (seeing, hearing, or feeling things that do not exist). If hallucinations occur, the patient should not drive or operate machinery.
If it is suspected that such symptoms may occur: do not drive, operate machinery, or perform tasks where drowsiness or falling asleep may put the patient (or others) at risk of serious injury or death. Such tasks should not be performed until the symptoms have resolved.
The doctor should be consulted if this situation is a problem for the patient.
Rolpryna SR contains lactose monohydrate
If the patient has intolerance to certain sugars, they should consult their doctor before taking the medicine.
3. How to take Rolpryna SR
This medicine should always be taken exactly as advised by the doctor or pharmacist. If there are any doubts, the doctor or pharmacist should be consulted.
Rolpryna SR is available in doses of 2 mg, 4 mg, and 8 mg.
Use in children and adolescents
Rolpryna SR should not be used in children.Rolpryna SR is not usually prescribed to patients under 18 years old.
Rolpryna SR may be used to treat symptoms of Parkinson's disease as a single medicine or in combination with another medicine called L-dopa (also known as levodopa). If the patient is taking L-dopa, they may experience involuntary movements (dyskinesia) when starting treatment with Rolpryna SR. The doctor should be informed if these symptoms occur, as it may be necessary to adjust the dose of the medicines being taken.
Rolpryna SR has a prolonged-release formulation, which ensures the release of ropinirole over a 24-hour period. If the patient has a condition that may cause the medicine to be removed from the body too quickly, such as diarrhea, the tablets may not dissolve completely and may not work properly. In such cases, the remains of the tablet may be visible in the stool. If this happens, the doctor should be contacted as soon as possible.
Page 3 7
What doses of Rolpryna SR should be taken?
Determining the correct dose of Rolpryna SR may take time.
The recommended initial doseof Rolpryna SR is 2 mg once daily for the first week. The doctor may increase the dose of Rolpryna SR to 4 mg once daily from the second week of treatment. If the patient is elderly, the doctor may increase the dose more slowly.
Then, the doctor may adjust the dose until the optimal dose for the patient is reached.
Some patients may take up to 24 mg of Rolpryna SR per day.
If the patient experiences intolerable side effects at the start of treatment, they should inform their doctor. The doctor may recommend changing the treatment to a lower dose of ropinirole in the form of immediate-release tablets, which the patient will take three times a day.
Do not take more than the recommended dose of Rolpryna SR.
It may take several weeks for the therapeutic effect of the medicine to occur.
Taking the dose of Rolpryna SR
Rolpryna SR should be taken once a dayat the same time every day.
The prolonged-release tablet(s) of Rolpryna SR should be swallowed whole, with a glass of water.
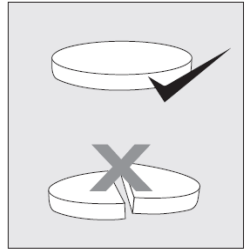
The prolonged-release tablet(s) of Rolpryna SR should not be divided, chewed, or crushed. If this happens, there is a risk of overdose due to the rapid release of the medicine in the body.
In case of switching treatment in patients taking ropinirole in the form of immediate-release tablets
The doctor will determine the dose of Rolpryna SR, prolonged-release tablets based on the previously taken dose of ropinirole in the form of immediate-release tablets.
The previously taken dose of ropinirole in the form of immediate-release tablets should be taken on the day before switching treatment. The next morning, Rolpryna SR, prolonged-release tablets should be taken, and no more ropinirole in the form of immediate-release tablets should be taken.
Taking more than the recommended dose of Rolpryna SR
Immediate medical attention should be sought.If possible, the packaging of Rolpryna SR should be shown.
If a person has taken more than the recommended dose of Rolpryna SR, they may experience nausea, vomiting, dizziness (feeling of spinning), drowsiness, mental or physical fatigue, fainting, or hallucinations.
Missing a dose of Rolpryna SR
A larger amount of prolonged-release tablets or a double dose should not be taken to make up for a missed dose.
If a dose of Rolpryna SR is missed for a day or longer, the doctor should be consulted about restarting treatment with Rolpryna SR.
Stopping treatment with Rolpryna SR
Treatment with Rolpryna SR should not be stopped without consulting the doctor.
Rolpryna SR should be taken for as long as the doctor recommends. Treatment should not be stopped unless the doctor advises it.
Page 4 7
In case of sudden stopping of treatment with Rolpryna SR, the symptoms of Parkinson's disease may worsen rapidly. Sudden stopping of treatment with Rolpryna SR may lead to a condition called malignant neuroleptic syndrome, which can cause serious health risks. Its symptoms include: akinesia (inability to move), muscle stiffness, fever, blood pressure fluctuations, tachycardia (increased heart rate), disorientation, decreased level of consciousness (e.g., coma).
If it is necessary to stop treatment with Rolpryna SR, the doctor will gradually reduce the dose being taken.
If there are any further doubts about taking this medicine, the doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, Rolpryna SR can cause side effects, although not everybody gets them.
The side effects of Rolpryna SR may occur most frequently during the start of treatment or shortly after increasing the dose. These side effects are usually mild and become less troublesome after a short period of taking the medicine. If there are any concerns about side effects, the doctor should be consulted.
Very common side effects (may affect more than 1 in 10 people)
- fainting
- feeling drowsy
- nausea
Common side effects (may affect up to 1 in 10 people)
- sudden sleepiness without warning (sudden sleep attacks)
- hallucinations (seeing things that are not real)
- vomiting
- dizziness (feeling of spinning)
- heartburn
- abdominal pain
- constipation
- swelling of the legs, feet, or hands.
Uncommon side effects (may affect up to 1 in 100 people)
- dizziness or fainting, especially when changing position from lying down or sitting to standing (related to low blood pressure)
- low blood pressure (hypotension)
- very strong feeling of drowsiness during the day (uncontrollable drowsiness)
- mental disorders, such as confusion (severe disorientation), delusions (irrational thoughts), or paranoia (unfounded suspicion)
- hiccups.
Some patients may experience the following side effects (frequency not known: cannot be estimated from the available data)
- allergic reactions such as red, itchy swellingon the skin (hives), swelling of the face, lips, mouth, tongue, or throat, which may cause difficulty swallowing or breathing, rashor severe itching (see section 2),
- changes in liver function, which have been shown in blood tests,
- aggressive behavior,
Page 5 7
- abuse of Rolpryna SR (desire to take higher doses of dopaminergic medicines than required to control motor symptoms, called dopamine dysregulation syndrome),
- inability to resist the impulse, drive, or temptation to engage in activities that may harm the patient or others, including behaviors such as:
- strong urge to gamble excessively despite significant personal or family consequences;
- changed or increased interest in sex and behaviors that significantly disturb the patient or others, such as increased sexual drive;
- uncontrolled excessive shopping or spending money;
- uncontrollable appetite (eating large amounts of food in a short time) or compulsive eating (eating more food than usual and more than needed to satisfy hunger).
- depression, apathy, anxiety, fatigue, excessive sweating, or pain (known as dopamine agonist withdrawal syndrome or DAWS) may occur after stopping treatment or reducing the dose of ropinirole. If these symptoms persist for more than a few weeks, the doctor may modify the treatment.
- episodes of overactivity, euphoria, or irritability
- spontaneous erections.
The doctor should be told if the patient experiences such behaviors; the doctor will discuss ways to treat or reduce these symptoms.
Taking Rolpryna SR with L-dopa
In patients taking Rolpryna SR in combination with L-dopa, other side effects may occur after some time:
- involuntary movements (dyskinesia) are a very common side effect. If the patient is taking L-dopa, they may experience involuntary movements (dyskinesia) when starting treatment with Rolpryna SR. The doctor should be informed if these symptoms occur, as it may be necessary to reduce the dose of the medicines being taken.
- feeling disoriented (common side effect).
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the doctor, pharmacist, or nurse should be informed. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: 22 49-21-301
Fax: 22 49-21-309
Website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Rolpryna SR
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
The medicine should not be stored at temperatures above 30°C.
The medicine should be stored in its original packaging to protect it from moisture.
Page 6 7
Medicines should not be disposed of via wastewater or household waste. The pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Rolpryna SR contains
- The active substance of Rolpryna SR is ropinirole. Each prolonged-release tablet contains 8 mg of ropinirole (in the form of ropinirole hydrochloride).
- The medicine also contains: hypromellose type 2208, lactose monohydrate, colloidal anhydrous silica, carbomers 4000-11000 mPas, hydrogenated castor oil, magnesium stearate in the tablet core, and hypromellose type 2910, titanium dioxide (E 171), macrogol 400, iron oxide red (E 172), iron oxide yellow (E 172), iron oxide black (E 172) in the coating.
What Rolpryna SR looks like and what the packaging contains
The prolonged-release tablets are brown-red, biconvex, and oval.
Packaging: 28 or 84 prolonged-release tablets in blisters, in a cardboard box.
For more detailed information, the marketing authorization holder or parallel importer should be consulted.
Marketing authorization holder in Italy, in the country of export:
KRKA, d.d., Novo mesto, Šmarješka cesta 6, Novo mesto, Slovenia
Manufacturer:
KRKA, d.d., Novo mesto, Šmarješka cesta 6, Novo mesto, Slovenia
TAD Pharma GmbH, Heinz-Lohmann-Str. 5, 27472 Cuxhaven, Germany
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Marketing authorization number in Italy, in the country of export: 040525192
040525230
Parallel import authorization number: 89/24
This medicine is authorized in the Member States of the European Economic Area under the following names:
Date of leaflet approval: 07.03.2024
[Information about the trademark]
| Bulgaria, Estonia, Spain, Lithuania, Latvia, Slovakia, Slovenia | Rolpryna SR |
| Czech Republic | Rolpryna |
| Norway, Portugal | Ropinirole Krka |
| Romania | Rolpryna EP |
Page 7 7
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Krka, d.d., Novo mesto
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Rolprina SrDosage form: Tablets, 0.25 mgActive substance: ropiniroleManufacturer: Glaxo Wellcome S.A.Prescription requiredDosage form: Tablets, 0.5 mgActive substance: ropinirolePrescription requiredDosage form: Tablets, 2 mgActive substance: ropiniroleManufacturer: Glaxo Wellcome S.A.Prescription required
Alternatives to Rolprina Sr in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Rolprina Sr in Spain
Alternative to Rolprina Sr in Ukraine
Online doctors for Rolprina Sr
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Rolprina Sr – subject to medical assessment and local rules.







