Pulmicort Turbuhaler

Ask a doctor about a prescription for Pulmicort Turbuhaler

How to use Pulmicort Turbuhaler
Leaflet accompanying the packaging: patient information
Pulmicort Turbuhaler, 100 μg/inhalation dose, inhalation powder
Pulmicort Turbuhaler, 200 μg/inhalation dose, inhalation powder
Budesonide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Pulmicort Turbuhaler and what is it used for
- 2. Important information before using Pulmicort Turbuhaler
- 3. How to use Pulmicort Turbuhaler
- 4. Possible side effects
- 5. How to store Pulmicort Turbuhaler
- 6. Contents of the packaging and other information
1. What is Pulmicort Turbuhaler and what is it used for
Budesonide, the active substance of Pulmicort Turbuhaler, belongs to a group of medicines called glucocorticosteroids. These medicines have a strong local anti-inflammatory effect. Pulmicort Turbuhaler is used in:
- bronchial asthma,
- chronic obstructive pulmonary disease (COPD).
2. Important information before using Pulmicort Turbuhaler
When not to use Pulmicort Turbuhaler
- if you are allergic (hypersensitive) to budesonide.
Warnings and precautions
Before starting treatment with Pulmicort Turbuhaler, tell your doctor if:
- you have pneumonia,
- you have a cold or chest infection or have breathing problems,
- you have ever had tuberculosis,
- you have liver problems,
- you have other diseases or conditions besides asthma.
Tell your doctor about any planned stressful situations (e.g., exams) or planned surgeries. Your doctor may then consider increasing the dose of oral glucocorticosteroids. If you experience blurred vision or other vision disturbances, contact your doctor.
Children and adolescents
The medicine is intended for use in the treatment of bronchial asthma in children aged 6 and older. Regular monitoring of growth in children and adolescents taking glucocorticosteroids is recommended, regardless of the route of administration.
Using Pulmicort Turbuhaler in patients with kidney or liver function disorders
Tell your doctor if you have ever had liver function disorders.
Pulmicort Turbuhaler and other medicines
Tell your doctor if you have had any worrying reactions after taking other medicines. Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take, including those available without a prescription and herbal preparations. Pulmicort Turbuhaler may affect the way some medicines work, and some medicines may affect the way Pulmicort Turbuhaler works. In particular, tell your doctor if you are taking:
- steroid medicines, including estrogen or steroid contraceptives,
- medicines used to treat fungal infections (such as ketoconazole and itraconazole),
- HIV protease inhibitors (such as ritonavir and nelfinavir).
Pregnancy and breastfeeding
Pregnancy If you are pregnant, think you may be pregnant, or plan to have a child, consult your doctor before using this medicine. If you become pregnant while taking Pulmicort Turbuhaler, tell your doctor as soon as possible. Breastfeeding Consult your doctor before using the medicine.
Driving and using machines
Pulmicort Turbuhaler does not affect the ability to drive or use machines.
3. How to use Pulmicort Turbuhaler
- Always use this medicine exactly as your doctor has told you. If you are not sure, ask your doctor.
Bronchial asthma
The dosage of Pulmicort Turbuhaler is individual. The following are general recommendations for determining the total initial dose, the maximum total dose of Pulmicort Turbuhaler, depending on previous asthma treatment and the patient's age. As with other inhaled medicines, a paradoxical bronchospasm may occur immediately after using Pulmicort Turbuhaler. If a severe reaction occurs, consult your doctor immediately. You should also consult your doctor if your symptoms do not improve despite regular use of the recommended doses.
Children aged 6 and older
- 100 μg to 800 μg per day, in 2 to 4 divided doses.
- If the total daily dose is not more than 400 μg, it can be administered once a day.
Adults
- The usual total daily dose is 200 μg to 800 μg and can be administered in 2 to 4 divided doses.
- In severe cases, the total daily dose may be up to 1600 μg.
- If the total daily dose is not more than 400 μg, it can be administered once a day.
It is recommended to use the smallest effective maintenance dose. Due to the very small amount of inhaled powder, you may not feel the taste of the medicine after inhalation. Improvement in clinical condition after administration of one dose of Pulmicort Turbuhaler can be expected after a few hours of inhalation. The full therapeutic effect is achieved after several weeks of treatment. Pulmicort Turbuhaler is intended for long-term treatment and does not provide quick relief of acute asthma attacks. When switching from oral glucocorticosteroids to inhaled treatment, the patient should be in a stable condition. For 10 days, it is recommended to use high doses of Pulmicort Turbuhaler in combination with the previously used oral glucocorticosteroid. Then, the dose of the oral glucocorticosteroid should be gradually reduced. Often, the use of oral glucocorticosteroids can be completely stopped.
Chronic obstructive pulmonary disease (COPD)
- The recommended dose of Pulmicort Turbuhaler is 400 μg administered twice a day.
In the case of patients with COPD who are taking oral glucocorticosteroids and are prescribed Pulmicort Turbuhaler, the same recommendations should be followed as for "Bronchial asthma". If COPD symptoms worsen, the patient should contact their doctor, who will prescribe appropriate treatment. If there is no noticeable improvement after using short-acting bronchodilators or if they need to be used more frequently than usual, consult your doctor.
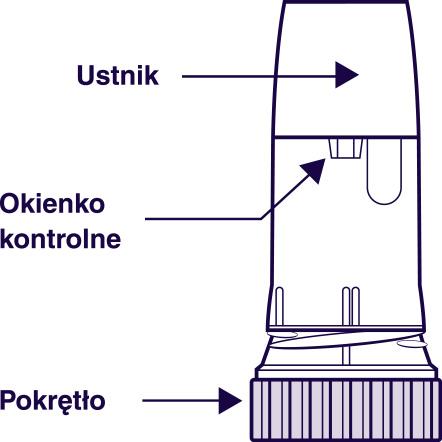
Preparing the Pulmicort Turbuhaler inhaler for the first use
Before the firstuse of a newPulmicort Turbuhaler inhaler, prepare it for use as follows:
- Remove the cap from the inhaler. When unscrewing, you may hear a characteristic rattling sound.
- Hold the Pulmicort Turbuhaler inhaler upright, with the turning grip at the bottom.
- Turn the grip in one direction until it clicks, then turn it in the opposite direction until it clicks (the direction you start turning the grip does not matter). You will hear a characteristic click. Repeat this operation, turning the grip in both directions.
- The Pulmicort Turbuhaler inhaler is now ready for use.
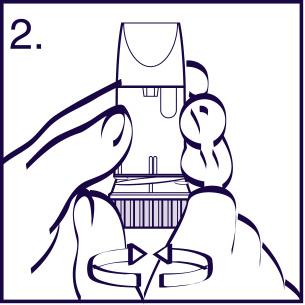
Using the inhaler
To perform an inhalation, follow the instructions below each time. The Turbuhaler is a multi-dose inhaler for administering the medicine.
When inhaling through the Turbuhaler, the powder is released into the lungs. Therefore, it is very important to perform a full, deep inhalation through the mouthpiece. Follow the instructions below.
- 1. Remove the cap from the inhaler.
- 2. Hold the inhaler upright, with the base at the bottom (Figure 2). When loading the dose, do not hold the inhaler by the mouthpiece. To load the dose, turn the grip as far as it will go in one direction, then turn it as far as it will go in the opposite direction(the direction you start turning the grip does not matter). You will hear a characteristic click. The inhaler is now loaded and ready for use, and you should not repeat this operation. To take the dose, follow the rest of the instructions.
- 3. Exhale. Do notexhale through the inhaler.
- 4. Place the mouthpiece between your teeth, close your lips, and perform a deep and forceful inhalation through your mouth. Do not chew or bite the mouthpiece. Do not use a damaged inhaler or an inhaler without a mouthpiece.
- 5. Before exhaling, remove the inhaler from your mouth. If more than one dose is prescribed, repeat the steps according to points 2-5.
- 6. Put the cap back on the inhaler and screw it on.
- 7. Rinse your mouth with water after taking the prescribed dose.
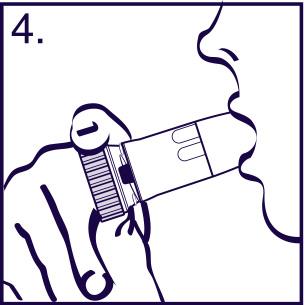
Warning!
Never EXHALEthrough the mouthpiece. After taking the medicine, always screw the cap on tightly. Due to the very small amount of powder, you may not feel the taste of the medicine after inhalation. However, you can be sure that the prescribed dose has been taken if you follow the instructions above.
Cleaning the Pulmicort Turbuhaler inhaler
Regularly clean the outside of the mouthpiece with a dry cloth (once a week).
Do not use water to clean the mouthpiece.
When to start using a new inhaler
Dose indicator When the red mark first appears in the control window, it means that there are about 20 doses left in the device (Figure 1). When the red band reaches the bottom of the control window (Figure 2), it means that the inhaler can no longer be used. The dose of medicine delivered from the inhaler may then be too small. When shaking the inhaler, you can hear a characteristic rattling sound of the desiccant.


Figure 1 Figure 2 If you feel that the effect of Pulmicort Turbuhaler is too strong or too weak, consult your doctor.
Using a higher than recommended dose of Pulmicort Turbuhaler
It is important to use the medicine as recommended by your doctor. Do not increase or decrease the dose of the medicine without consulting your doctor. If you take more than the recommended dose, consult your doctor immediately.
Missing a dose of Pulmicort Turbuhaler
Pulmicort Turbuhaler inhalation powder should be taken as recommended by your doctor and only the prescribed number of doses should be taken. Do not take a double dose to make up for a missed dose. If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Pulmicort Turbuhaler can cause side effects, although not everybody gets them.
Stop using Pulmicort Turbuhaler and consult your doctor immediately if you experience:
the following symptoms:severe allergic reactions, e.g., swelling of the face, especially around the mouth (tongue and/or throat) and/or difficulty swallowing or hives occurring at the same time as difficulty breathing (angioedema), bronchospasm, and/or sudden weakness (fainting).
Other side effects
Common side effects (less than 1 in 10 people)
- fungal infections (candidiasis) of the mouth and throat. Rinse your mouth with water after taking each dose to reduce the risk of oral candidiasis.
- irritation of the throat, cough, hoarseness,
- loss of voice,
- pneumonia (lung infection) in patients with COPD.
Tell your doctor if you experience any of the following symptoms, which may be signs of a lung infection:
- fever or chills,
- increased production of mucus, change in mucus color,
- worsening cough or increased breathing difficulties.
Uncommon side effects (less than 1 in 100 people)
- cataract,
- blurred vision,
- muscle cramps,
- muscle tremors,
- depression,
- anxiety.
Rare side effects (less than 1 in 1000 people)
- immediate and delayed hypersensitivity reactions, including rash, contact dermatitis, urticaria, angioedema (swelling of soft tissues caused by increased vascular permeability: swelling of the eyelids, face, lips, tongue, and/or larynx), severe allergic reactions,
- easy bruising (bruises),
- bronchospasm,
- nervousness, changes in behavior (mainly in children).
- systemic effects of glucocorticosteroids, including suppression of adrenal function and growth retardation (in children and adolescents),
- hoarseness (in children),
- hoarseness (in children).
Side effects with unknown frequency
- glaucoma,
- sleep disturbances,
- anxiety,
- excessive psychomotor activity,
- aggression.
The possible systemic effects include Cushing's syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, decreased bone density, cataract, glaucoma, and more rarely, psychiatric or behavioral problems, including excessive psychomotor activity, sleep disturbances, anxiety, depression, or aggression (especially in children). If you experience any of these side effects, consult your doctor or go to the nearest hospital immediately.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Pulmicort Turbuhaler
Store at a temperature not exceeding 30°C. Keep the medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the packaging after the expiry date (EXP). The expiry date refers to the last day of the month stated. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Pulmicort Turbuhaler contains
The active substance is budesonide. One dose contains 100 micrograms (μg) or 200 micrograms (μg) of budesonide.
What Pulmicort Turbuhaler looks like and what the pack contains
A dose counter with a mouthpiece and protective cap in a cardboard box. Pulmicort Turbuhaler 100 μg contains 200 doses. Pulmicort Turbuhaler 200 μg contains 100 doses. Each inhaler is also labeled with: 200 DOSES BUDESONIDE 100 μg/DOSE LOT/CH.-B/LOTE ZM 1234 EXP/VERW.BIS/CAD MM-YYYY or 100 DOSES BUDESONIDE 200 μg/DOSE LOT/CH.-B/LOTE ZM 1234 EXP/VERW.BIS/CAD MM-YYYY
Marketing authorization holder and manufacturer
Marketing authorization holder: AstraZeneca AB, S-151 85 Sodertalje, Sweden. Manufacturer: AstraZeneca AB, Forskargatan 18, 151 36 Södertälje, Sweden. For more detailed information, contact the representative of the marketing authorization holder: AstraZeneca Pharma Poland Sp. z o.o., ul. Postępu 14, 02-676 Warsaw, tel: +48 22 245 73 00, fax: +48 22 485 30 07. Date of last revision of the leaflet:November 2022
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAstraZeneca AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Pulmicort TurbuhalerDosage form: Suspension, 0.125 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.25 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.5 mg/mlActive substance: budesonidePrescription required
Alternatives to Pulmicort Turbuhaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Pulmicort Turbuhaler in Ukraine
Alternative to Pulmicort Turbuhaler in Spain
Online doctors for Pulmicort Turbuhaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Pulmicort Turbuhaler – subject to medical assessment and local rules.