

Prospan

Ask a doctor about a prescription for Prospan

How to use Prospan
PATIENT INFORMATION LEAFLET
1 (6)
Leaflet accompanying the packaging: patient information
Prospan, 35 mg / 5ml, oral liquid
Hederae helicis folii extractum siccum
Dry extract of ivy leaves
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
This medicine should always be taken exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, so you can read it again if you need to.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should tell their doctor or pharmacist. See section 4.
- If there is no improvement or the patient feels worse after 7 days, they should contact their doctor.
Table of contents of the leaflet:
- 1. What is Prospan and what is it used for
- 2. Important information before taking Prospan
- 3. How to take Prospan
- 4. Possible side effects
- 5. How to store Prospan
- 6. Contents of the pack and other information
1. What is Prospan and what is it used for
Prospan is a herbal medicine containing dry extract of ivy leaves used as an expectorant in productive cough (so-called wet cough).
This medicine belongs to the group of expectorants used to help remove mucus and phlegm from the airways in productive cough (so-called wet cough).
If there is no improvement or the patient feels worse after 7 days, or if symptoms such as shortness of breath, fever, purulent or bloody sputum appear, they should consult their doctor immediately.
2. Important information before taking Prospan
When not to take Prospan:
2 (6)
Warnings and precautions
Before starting to take Prospan, consult a doctor or pharmacist.
If symptoms persist or appear, such as shortness of breath, fever, purulent or bloody sputum, consult a doctor immediately.
Concomitant use of cough suppressants, such as codeine or dextromethorphan, is not recommended without consulting a doctor.
Care should be taken when using in patients with stomach or duodenal ulcers.
Children
Prospan is not intended for use in children under 6 years of age.
Prospan and other medicines
Tell your doctor or pharmacist about all medicines you are taking now or have taken recently, as well as any medicines you plan to take, including those available without a prescription.
No studies have been conducted. There are no known interactions to date.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before taking this medicine.
This medicine should not be taken during pregnancy and breastfeeding, as sufficient studies have not been conducted in pregnant and breastfeeding women.
Driving and using machines
No special precautions are necessary.
Prospan contains sorbitol and glucose (maltodextrin component in the aroma).
One sachet contains 2.7 g of liquid crystallizable sorbitol, which corresponds to 1.9 g of sorbitol (E420).
If the patient has an intolerance to some sugars, they should consult their doctor.
Patients with hereditary fructose intolerance should not take this medicinal product.
Sorbitol is a source of fructose. If the patient (or their child) has previously been diagnosed with an intolerance to some sugars or has been diagnosed with hereditary fructose intolerance, a rare genetic disease in which the patient's body does not break down fructose, they should consult their doctor before taking the medicine or giving it to their child.
3. How to take Prospan
This medicine should always be taken as directed by a doctor or pharmacist. If in doubt, consult a doctor or pharmacist.
3 (6)
Unless otherwise directed by a doctor, the usual dose is:
Adults and adolescents over 12 years of age:take the liquid from 1 sachet (5 ml) 3 times a day.
The daily dose should not exceed 3 sachets per day (which corresponds to 105 mg of dry extract of ivy leaves).
Children from 6 to 12 years of age:take the liquid from 1 sachet (5 ml) 2 times a day (which corresponds to 70 mg of dry extract of ivy leaves).
The daily dose should not exceed 2 sachets per day (which corresponds to 70 mg of dry extract of ivy leaves).
Method of administration
For oral use.
The undiluted liquid should be taken in the morning, at lunchtime, and in the evening.
Do not take in a lying position.
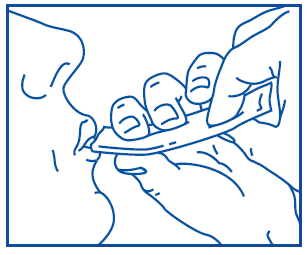
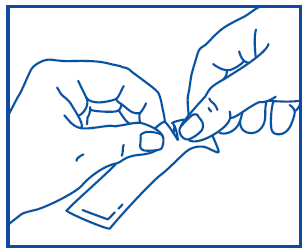
Prospan oral liquid is packaged in a sachet that is easy to open, and its contents are also easy to take. For more information on using sachets, refer to the following diagram:
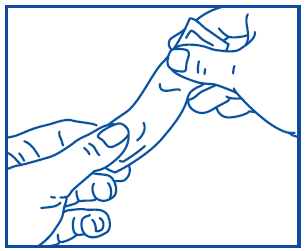
Gently knead the sachet before use, as shown.
To open, tear off the end of the sachet, holding it firmly.
Drink the medicine by squeezing the sachet until it is empty.
Duration of treatment
If symptoms persist for more than one week while taking the medicinal product, consult a doctor.
If, despite taking the medicine for more than 1 week, symptoms persist, there is no improvement, or the patient feels worse, or symptoms such as shortness of breath, fever, purulent or bloody sputum appear, consult a doctor immediately.
If you feel that the effect of Prospan is too strong or too weak, consult a doctor or pharmacist.
Taking a higher dose of Prospan than recommended
Do not exceed the recommended daily dose. If a significantly higher dose of the medicine is taken than recommended (more than three times the daily dose), nausea, vomiting, or diarrhea may occur. In such a case, consult a doctor.
Missing a dose of Prospan
Do not take a double dose to make up for a missed dose, but continue taking the medicine as directed by a doctor or as recommended in this leaflet.
4 (6)
If you have any further doubts about taking this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following frequency classification is used for the evaluation of side effects:
Unknown:(frequency cannot be estimated from the available data).
Unknown frequency:
After taking medicines containing ivy leaf extract, allergic reactions may occur, such as:
hives, rash, shortness of breath, swelling, redness, and itching of the skin.
Unknown frequency:
Gastrointestinal disorders, such as:
nausea, vomiting, diarrhea.
Stop taking Prospan if the first symptoms of hypersensitivity appear.
If you experience any of these or other side effects not listed in this leaflet, inform your doctor.
Reporting side effects
If you experience any side effects, including any possible side effects not listed in the leaflet, tell your doctor or pharmacist, or nurse.
Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Prospan
Keep the medicine out of the sight and reach of children.
There are no special precautions for storing the medicinal product.
Do not use the medicine after the expiry date stated on the carton and sachet after EXP.
The expiry date refers to the last day of the given month.
5 (6)
6. Contents of the pack and other information
What Prospan contains
- The active substance of Prospan is dry extract of ivy leaves Hederae helicis folii extractumsiccum.
- One sachet contains 35 mg of extract (in the form of dry extract) from Hedera helix L., folium(ivy leaf) (5-7.5:1), extraction solvent: ethanol 30% (m/m).
- The other ingredients are: Purified water, Potassium sorbate, Anhydrous citric acid, Liquid crystallizable sorbitol (E420), Xanthan gum, Natural peppermint aroma Frescofort Permaseal (composition: natural flavoring substance, corn maltodextrin, modified corn starch), Natural orange aroma (composition: orange essential oil, ethanol), Levomenthol. 5 ml of liquid (one sachet) contains 1.9 g of sorbitol (Ph.Eur.) (sweetening agent) = 0.16 bread units.
What Prospan looks like and contents of the pack
Prospan oral liquid is available in original packaging of 21 and 30 sachets of 5 ml oral liquid.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Engelhard Arzneimittel GmbH & Co. KG
Herzbergstr. 3
61138 Niederdorfelden
Germany
To obtain more detailed information about the medicine, contact the representative of the marketing authorization holder:
Salveo Poland Sp. z o.o.
Okrężna 83, 83a lok.5
02-933 Warsaw
e-mail: [email protected]
Date of last revision of the leaflet:
Warning:
Prospan contains an active substance of plant origin. The plant extract may sometimes cause slight cloudiness or a slight change in the taste of Prospan. However, this does not affect the effectiveness and quality of the product.
6 (6)
- Country of registration
- Prescription requiredNo
- Manufacturer
- ImporterEngelhard Arzneimittel GmbH & Co. KG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ProspanDosage form: Solution, 20 mg/mlActive substance: acetylcysteinePrescription not requiredDosage form: Tablets, 100 mgActive substance: acetylcysteinePrescription not requiredDosage form: Powder, 100 mg/sachetActive substance: acetylcysteineManufacturer: Salutas Pharma GmbHPrescription not required
Online doctors for Prospan
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Prospan – subject to medical assessment and local rules.














