

Propranolol Vzf


How to use Propranolol Vzf
Leaflet attached to the packaging: patient information
PROPRANOLOL WZF, 1 mg/ml, solution for injection
Propranolol hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Propranolol WZF and what is it used for
- 2. Important information before using Propranolol WZF
- 3. How to use Propranolol WZF
- 4. Possible side effects
- 5. How to store Propranolol WZF
- 6. Contents of the packaging and other information
1. What is Propranolol WZF and what is it used for
Propranolol WZF belongs to a group of medicines called beta-blockers (beta-adrenergic receptor blockers).
The medicine in the form of a solution for injection is used in acute conditions requiring immediate treatment - heart rhythm disorders, thyroid crisis.
2. Important information before using Propranolol WZF
When not to use Propranolol WZF:
- if the patient is allergic to propranolol or any of the other ingredients of this medicine (listed in section 6),
- if the patient has asthma and experiences bronchial spasms,
- if the patient has bradycardia (slow heart rate),
- if the patient has cardiogenic shock,
- if the patient has uncontrolled heart failure,
- if the patient has arterial hypotension,
- if the patient has metabolic acidosis,
- if the patient has been fasting for a long time,
- if the patient has severe peripheral circulation disorders,
- if the patient has second or third degree atrioventricular block,
- if the patient has Prinzmetal's angina,
- if the patient has sick sinus syndrome,
- if the patient has an untreated pheochromocytoma.
The medicine should not be used in patients with a tendency to hypoglycemia (low blood glucose levels), e.g. malnourished, with chronic liver diseases, diabetes, taking medications that inhibit the reaction to catecholamines (e.g. beta-blockers).
Warnings and precautions
Before starting treatment, discuss it with your doctor or pharmacist, or nurse.
Particular caution should be exercised when using Propranolol WZF
- In patients with controlled heart failure, with first degree atrioventricular block;
- In patients with pheochromocytoma during concomitant use of alpha-blockers.
- When using Propranolol WZF, do not take calcium channel blockers, such as verapamil, diltiazem, as this may enhance their effect, especially in patients with left ventricular failure and/or atrioventricular conduction disorders. This may cause severe arterial hypotension, bradycardia, and heart failure. Do not administer beta-blockers and calcium channel blockers intravenously at the same time. Do not administer beta-blockers intravenously within 48 hours of discontinuing calcium channel blockers, and do not administer calcium channel blockers intravenously within 48 hours of discontinuing beta-blockers.
- The medicine may exacerbate peripheral circulation disorders.
- The medicine may mask or modify the symptoms of hypoglycemia (especially heart rate acceleration), and may even cause a decrease in blood glucose levels in patients without diabetes (e.g. newborns, children, patients undergoing hemodialysis).
- The medicine may mask the symptoms of hyperthyroidism.
- The medicine may slow down the heart rate, which is a result of its properties - if these symptoms worsen, the doctor will reduce the dose of propranolol.
- During treatment with Propranolol WZF, severe allergic reactions may occur, especially in people who have had an allergy to this type of medicine in the past.
- Propranolol interferes with the determination of bilirubin in serum by the diazo method and catecholamines by the fluorescence method.
- In patients with renal and/or hepatic impairment, caution should be exercised, especially when starting treatment and selecting the initial dose. Propranolol should be used with caution in patients with liver cirrhosis. In patients with portal hypertension, liver function may worsen, and even hepatic encephalopathy may develop.
- The medicine should be used with caution in elderly patients. Treatment should be started with the smallest dose. The optimal dose should be determined individually, depending on the patient's overall condition and their reaction to propranolol.
Propranolol WZF and other medicines
Tell your doctor or pharmacist about all the medicines you are taking, have recently taken, or plan to take.
Propranolol may affect the action of some medicines, and some medicines may affect the action of propranolol.
Especially, inform your doctor about taking any of the following medicines:
- verapamil, diltiazem, nifedipine, nisoldipine, nicardipine, isradipine, lacidipine (medicines used to treat high blood pressure or angina pectoris);
- disopyramide, lidocaine, quinidine, propafenone (medicines used to treat heart rhythm disorders);
- adrenaline (a medicine that stimulates heart function);
- ibuprofen and indomethacin (anti-inflammatory and analgesic medicines);
- ergotamine and dihydroergotamine (medicines used to treat migraines);
- chlorpromazine and thioridazine (medicines used in psychiatry);
- cimetidine (a medicine used to treat stomach and duodenal ulcers);
- rifampicin (an antibiotic);
- theophylline (an anti-asthmatic medicine);
- acenocoumarol, warfarin (anticoagulant medicines);
- hydralazine (a medicine used to treat high blood pressure).
Caution should be exercised when administering propranolol and antidiabetic medicines, as propranolol may enhance their hypoglycemic effect.
In the event of concomitant use of propranolol and clonidine (a medicine used to treat high blood pressure or migraines) and the need to discontinue one of these medicines, propranolol should be discontinued a few days before discontinuing clonidine.
When replacing clonidine with propranolol, it should be introduced into treatment a few days after discontinuing clonidine. Do not discontinue these medicines without consulting a doctor.
Anesthetics used with propranolol may increase the risk of hypertension. If a patient taking propranolol is scheduled for surgery, they should inform the anesthesiologist about taking propranolol.
Propranolol WZF and alcohol
Alcohol weakens the effect of propranolol.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
The medicine can be used during pregnancy only when necessary.
Like other beta-blockers, propranolol may cause complications in the fetus, miscarriage, or premature birth. Side effects may also occur, especially decreased blood glucose levels and bradycardia in the fetus or newborn. The risk of cardiopulmonary complications in newborns during the postnatal period increases.
Propranolol passes into breast milk. Breastfeeding is not recommended during treatment with this medicine.
3. How to use Propranolol WZF
This medicine should always be used as directed by the doctor. In case of doubts, consult a doctor or pharmacist.
The medicine is intended for intravenous administration.
Adults
Initially, a dose of 1 mg (1 ml) is administered intravenously over 1 minute.
The dose can be repeated every 2 minutes until improvement is achieved or a maximum dose of 10 mg is reached in conscious patients, or a maximum dose of 5 mg in anesthetized patients.
Elderly patients
The medicine should be used with caution in elderly patients. Treatment should be started with the smallest dose. The optimal dose should be determined individually, depending on the patient's overall condition and their reaction to propranolol.
Use in children and adolescents
In some cases, Propranolol WZF may be used in children to treat arrhythmias (heart rhythm disorders). The doctor will adjust the dosage based on the child's age and body weight.
Instructions for opening the ampoule
Before opening the ampoule, make sure the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
A colored dot is placed on each ampoule (see Figure 1) as a mark indicating the break point below it.
- To open the ampoule, hold it vertically in both hands, with the colored dot facing each other - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
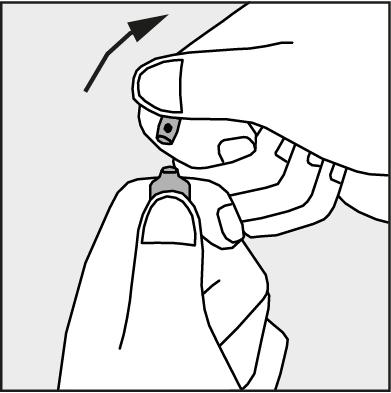
- Press according to the arrow shown in Figure 3. The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused product should be destroyed in accordance with applicable regulations.
Figure 1.
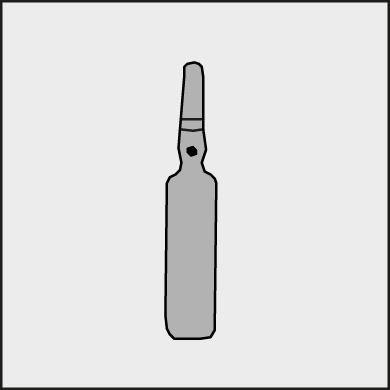
Figure 2.
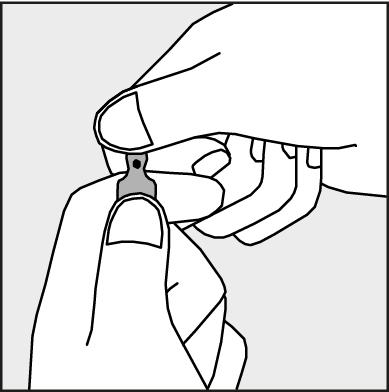
Figure 3.
Using a higher dose of Propranolol WZF than recommended
Symptoms: bradycardia, hypotension, acute heart failure, bronchospasm.
Treatment should include: close monitoring, treatment in the intensive care unit. Symptomatic treatment should be initiated.
Discontinuing Propranolol WZF
If it is necessary to discontinue propranolol, especially in patients with coronary heart disease, it is recommended to gradually reduce the doses of the medicine over 7 to 14 days. Do not stop the medicine abruptly! In the case of patients scheduled for surgical procedures, propranolol should be discontinued at least 24 hours before the procedure.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Propranolol WZF is usually well tolerated.
The following may occur:
Common (in 1 to 10 out of 100 people):
sleep disorders; nightmares; bradycardia; cold extremities; pallor, cyanosis, and then redness of the fingers, accompanied by pain and numbness (Raynaud's syndrome); feeling of fatigue and/or exhaustion (usually transient).
Uncommon (in 1 to 10 out of 1,000 people):
gastrointestinal disorders.
Rare (in 1 to 10 out of 10,000 people):
decreased platelet count; hallucinations; psychoses; mood changes; dizziness; paresthesia (feeling of tingling or numbness); disorientation, vision disturbances; dry eyes; worsening of heart failure; atrioventricular block; sudden drop in blood pressure with fainting; exacerbation of intermittent claudication (symptoms: calf pain occurring while walking);
bronchospasm (asthma attacks, wheezing, coughing) in patients with asthma or a history of asthma-like disorders (sometimes fatal); alopecia; purpura (bruises on the skin); psoriasis-like skin reactions; exacerbation of psoriasis symptoms (red, scaly skin lesions); rash.
Very rare (less than 1 in 10,000 people):
muscle weakness resembling myasthenia or exacerbation of myasthenia (isolated cases).
An increase in antinuclear antibody titers has been observed, although the clinical significance of this phenomenon is not explained.
Frequency not known (cannot be estimated from the available data):
decreased blood glucose levels (especially in children, elderly patients, patients undergoing dialysis, patients taking antidiabetic medicines, patients following a strict diet, patients with chronic liver disease); seizures related to decreased blood glucose levels (hypoglycemia).
In case of worsening side effects, tell your doctor, who will consider discontinuing the medicine (which should be done gradually, according to the doctor's instructions).
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
e-mail: ndl@urpl.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Propranolol WZF
Keep the medicine out of the sight and reach of children.
Store in a temperature not exceeding 25°C. Store the ampoules in the outer packaging to protect them from light.
Do not use this medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP indicates the expiry date, and after the abbreviation Lot indicates the batch number.
Medicines should not be disposed of via wastewater or household waste containers. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Propranolol WZF contains
- The active substance of the medicine is propranolol hydrochloride. Each ml contains 1 mg of propranolol hydrochloride.
- The other ingredients are: citric acid (to adjust pH), water for injections.
What Propranolol WZF looks like and what the packaging contains
Propranolol WZF is a solution for injection. The packaging contains 10 ampoules of 1 ml each.
Marketing authorization holder and manufacturer
Warsaw Pharmaceutical Works Polfa S.A.
Karolkowa 22/24 Street; 01-207 Warsaw
phone: 22 691 39 00
Date of the last update of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterWarszawskie Zakłady Farmaceutyczne POLFA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
Talk to a doctor online
Need help understanding this medicine or your symptoms? Online doctors can answer your questions and offer guidance.














