
Priorix-tetra

Ask a doctor about a prescription for Priorix-tetra

How to use Priorix-tetra
Leaflet attached to the packaging: information for the user
Priorix-Tetra, powder and solvent for solution for injection in a pre-filled syringe
Syringe
Measles, mumps, rubella and chickenpox vaccine, live
You should carefully read the contents of this leaflet before using the vaccine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
The content of this leaflet has been written in a way that is easy to understand, assuming that it will be read by the person receiving the vaccine. However, since this vaccine may be given to adults and children, it is possible that the content of the leaflet will be read by a parent or guardian of the child.
Table of contents of the leaflet
- 1. What is Priorix-Tetra vaccine and what is it used for
- 2. Important information before using Priorix-Tetra vaccine
- 3. How to use Priorix-Tetra vaccine
- 4. Possible side effects
- 5. How to store Priorix-Tetra vaccine
- 6. Contents of the pack and other information
1. What is Priorix-Tetra vaccine and what is it used for
Priorix-Tetra is a vaccine used in individuals from 11 months of age to prevent diseases caused by measles, mumps, rubella and chickenpox viruses. In certain circumstances, Priorix-Tetra may also be given to children from 9 months of age.
How Priorix-Tetra vaccine works
After administration of Priorix-Tetra vaccine, the immune system (the body's defense system) of the vaccinated person will start producing antibodies that will protect against infection with measles, mumps, rubella and chickenpox viruses.
Although Priorix-Tetra contains live viruses, they have been weakened to such an extent that they cannot cause measles, mumps, rubella and chickenpox in healthy individuals.
As with all vaccines, Priorix-Tetra may not fully protect all vaccinated individuals.
2. Important information before using Priorix-Tetra vaccine
When not to use Priorix-Tetra vaccine
- if you are allergic to any of the ingredients of this vaccine (listed in section 6). Allergic reactions may include an itchy rash, difficulty breathing, swelling of the face or tongue,
- if you have had an allergic reaction after previous administration of any measles, mumps, rubella and/or chickenpox vaccine,
- if you are allergic to neomycin (an antibiotic). Contact dermatitis caused by neomycin (a skin rash that occurs as a result of direct contact with the allergen, such as neomycin) should not be a problem, but you should first
talk to your doctor,
- if you have a severe infection with a fever. In this case, vaccination should be postponed until you have recovered. Mild infections, such as a cold, should not be a contraindication to vaccination, but you should first tell your doctor,
- if you have any disease (e.g. HIV infection or AIDS) or if you are taking medicines that may weaken your immune system. Whether or not you will receive the vaccine will depend on the level of your immune system,
- if you are pregnant. Additionally, you should avoid becoming pregnant for 1 month after vaccination.
Warnings and precautions
You should talk to your doctor or pharmacist before administering Priorix-Tetra vaccine if:
- you or your family have a history of seizures, including febrile seizures. In this case, you should be closely monitored after vaccination, as fever associated with vaccination may occur, especially between 5 and 12 days after vaccination (see section 4),
- you have ever had a severe allergic reaction to egg protein,
- you have had side effects after vaccination against measles, mumps or rubella, including easy bruising or bleeding longer than usual (see section 4),
- you have a weakened immune system (e.g. due to HIV infection). You should be closely monitored because the response to the vaccine may not be sufficient to provide protection against the disease (see section 2 "When not to use Priorix-Tetra vaccine").
If you are vaccinated within 72 hours of contact with a person with measles or chickenpox, Priorix-Tetra vaccine may provide some protection against the disease.
After vaccination, you should try to avoid close contact with:
- people with weakened immune systems,
- pregnant women who have not had chickenpox or have not been vaccinated against chickenpox,
- newborn babies of mothers who have not had chickenpox or have not been vaccinated against chickenpox.
After or even before administration of each vaccine injection, fainting (especially in adolescents) may occur. Therefore, you should inform your doctor or nurse if you have ever fainted during an injection.
As with other vaccines, Priorix-Tetra vaccine may not fully protect all vaccinated individuals. However, in vaccinated individuals, the course of the disease is usually much milder than in unvaccinated individuals.
Priorix-Tetra vaccine and other medicines
You should tell your doctor about all medicines you are taking or have recently taken, medicines you plan to take, and vaccines you have recently received.
Vaccination may be postponed by your doctor for at least 3 months if you have recently received a blood transfusion or human antibodies (immunoglobulin).
If you are to undergo a tuberculin test (a skin test for tuberculosis), it should be performed before or at the same time as vaccination, or after an interval of at least 6 weeks after vaccination.
You should avoid taking salicylates (substances found in many anti-fever and pain-relieving medicines) for 6 weeks after vaccination.
Priorix-Tetra vaccine can be given at the same time as other vaccines. Each vaccine should be given at a different site on the body.
Pregnancy and breastfeeding
Priorix-Tetra vaccine should not be given to pregnant women.
If you are pregnant or breastfeeding, or think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this vaccine. Additionally, it is important that for 1 month after vaccination, you do not become pregnant. During this time, you should use an effective method of contraception to avoid pregnancy.
Driving and using machines
There are no data to suggest that Priorix-Tetra vaccine affects the ability to drive and use machines.
Priorix-Tetra vaccine contains sorbitol, para-aminobenzoic acid, phenylalanine, sodium and potassium
This vaccine contains 14 mg of sorbitol per dose.
Priorix-Tetra vaccine contains para-aminobenzoic acid. It may cause allergic reactions (possible late reactions) and, exceptionally, bronchospasm.
The vaccine contains 583 micrograms of phenylalanine per dose. Phenylalanine may be harmful to patients with phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates in the body because the body does not eliminate it properly.
This vaccine contains less than 1 mmol (23 mg) of sodium per dose, i.e. the vaccine is considered "sodium-free".
This vaccine contains potassium, less than 1 mmol (39 mg) of potassium per dose, i.e. the vaccine is considered "potassium-free".
3. How to use Priorix-Tetra vaccine
Priorix-Tetra vaccine is given subcutaneously or intramuscularly, in the upper arm or outer thigh.
Priorix-Tetra vaccine is intended for individuals from 11 months of age.
The vaccination schedule and the number of doses to be given will be determined by your doctor based on official recommendations.
Vaccines should never be given intravascularly.
4. Possible side effects
As with any medicine, side effects may also occur after administration of this vaccine, although they may not occur in everyone.
After administration of the vaccine, the following side effects may occur:
♦
Very common (may occur more frequently than 1 in 10 doses of the vaccine):
- pain and redness at the injection site
- fever of 38°C or higher*
- swelling at the injection site, in the case of administration to adolescents and adults
♦
Common (may occur no more frequently than 1 in 10 doses of the vaccine):
- swelling at the injection site, in the case of administration to children
- fever higher than 39.5°C*
- irritability
- rash (spots and/or blisters)
♦
Uncommon (may occur no more frequently than 1 in 100 doses of the vaccine):
- unusual crying, nervousness, insomnia
- general feeling of being unwell, lethargy, fatigue
- swelling of the parotid glands (glands in the cheek area)
- diarrhea, vomiting
- loss of appetite
- upper respiratory tract infection
- cold
- swelling of the lymph nodes
♦
Rare (may occur no more frequently than 1 in 1,000 doses of the vaccine):
- middle ear infection
- febrile seizures
- cough
- bronchitis
* More frequent occurrence of fever has been observed after administration of the first dose of Priorix-Tetra vaccine compared to separate administration of measles, mumps, rubella and chickenpox vaccines during the same visit.
The following side effects have been reported during routine use of measles, mumps, and rubella vaccine or chickenpox vaccine manufactured by GlaxoSmithKline Biologicals:
- joint and muscle pain
- allergic reactions (allergic reactions). This condition may manifest as an itchy or blistering rash, swelling of the eyelids and face, difficulty breathing or swallowing, sudden drop in blood pressure and loss of consciousness. These reactions may occur before leaving the doctor's office. If you experience any of these symptoms, you should immediately see a doctor
- infection or inflammation of the brain, spinal cord, and peripheral nerves resulting in transient walking difficulties (ataxia) and/or transient loss of coordination, stroke, inflammation of some nerves with possible tingling or loss of sensation or movement disorders (Guillain-Barré syndrome)
- narrowing or obstruction of blood vessels
- small local bleeding or easier bruising due to decreased platelet count
- erythema multiforme (symptoms include red, often itchy spots, similar to a chickenpox-like rash, that appear on the limbs and sometimes on the face and other parts of the body)
- rash similar to chickenpox
- shingles
- symptoms similar to measles and mumps (including transient, painful swelling of the testicles and swollen lymph nodes in the neck)
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products:
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: 22 49 21 301
Fax: 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Priorix-Tetra vaccine
Store the vaccine out of sight and reach of children.
Do not use this vaccine after the expiry date stated on the label after "EXP".
The expiry date refers to the last day of the month stated.
The abbreviation "Lot" means the batch number of the vaccine.
Store and transport in a cool place (2°C - 8°C).
Do not freeze.
Store in the original packaging to protect from light.
After reconstitution, the vaccine should be used immediately or stored in a refrigerator (2°C - 8°C). If it is not used within 24 hours, it should be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Priorix-Tetra vaccine contains
The active substances of the vaccine are: live, attenuated measles, mumps, rubella and chickenpox viruses.
Other ingredients of the vaccine are:
Powder: amino acids (including phenylalanine), anhydrous lactose, mannitol (E 421), sorbitol (E 420),
medium 199 (including phenylalanine, para-aminobenzoic acid, sodium and potassium).
Solvent: water for injections.
What Priorix-Tetra vaccine looks like and contents of the pack
The vaccine is provided as a powder and solvent for solution for injection (powder in a 1-dose vial, solvent in a pre-filled syringe of 0.5 ml), with separate needles or without needles, in the following packs:
- with 2 separate needles: packs of 1 or 10,
- without needles: packs of 1 or 10.
Priorix-Tetra vaccine is a white to pale pink powder, some of which may be yellowish, and a clear, colorless solvent (water for injections) used to dissolve the powder.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
GlaxoSmithKline Biologicals S.A.
Rue de l’Institut 89
B-1330 Rixensart
Belgium
Date of last revision of the leaflet:March 2025
Other sources of information
Detailed information on this vaccine is available on the website of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products .
-------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
As with all vaccines given by injection, you should always ensure that you have the means to provide appropriate treatment and medical supervision in case of rare anaphylactic reactions after vaccination.
Alcohol and other disinfectants should evaporate from the skin before administering the vaccine, as they may inactivate the attenuated viruses in the vaccine.
Priorix-Tetra vaccine should never be given intravascularly or intradermally.
Because compatibility studies have not been performed, this medicinal product should not be mixed with other medicinal products.
After reconstitution, the vaccine should be inspected visually. Due to minor changes in pH, the vaccine after reconstitution may have a color ranging from light apricot to pink.After reconstitution, semi-transparent particles of the product may be visible. This is a normal phenomenon and does not affect the efficacy of the vaccine.
If the vaccine has a different color or contains other solid particles, it should not be administered.
The vaccine should be reconstituted by adding the entire solvent from the pre-filled syringe to the vial with the powder.
To attach the needle to the pre-filled syringe, you should carefully follow the instructions presented in the drawings 1 and 2. The pre-filled syringe provided with Priorix-Tetra vaccine may, however, differ slightly from the one shown in the drawing (it may not have a thread for screwing in the needle). In this case, the needle should be attached without screwing.
Needle
Pre-filled syringe
You should always hold the pre-filled syringe by its body, not by the plunger or the Luer Lock adapter (LLA), and the needle should be kept in line with the pre-filled syringe (as shown in drawing 2). Failure to follow these instructions may cause the LLA adapter to bend and leak from the pre-filled syringe.
If the LLA adapter comes off during attachment of the needle to the pre-filled syringe, you should use a new dose of the vaccine (a new pre-filled syringe and vial).
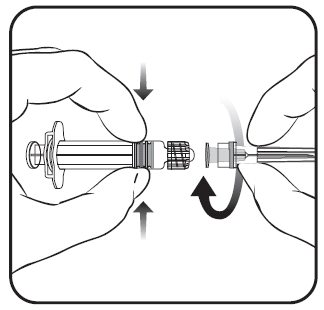
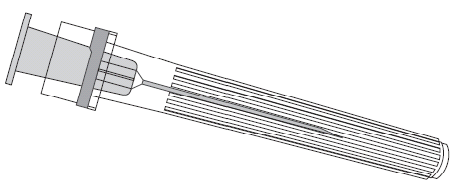
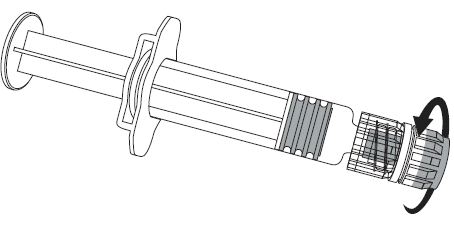
Figure 1. Figure 2.
- 1. You should unscrew the cap of the pre-filled syringe by turning it in the opposite direction to the arrow (as shown in figure 1).
Regardless of whether the LLA adapter rotates or not, you should follow the instructions below:
- 2. You should attach the needle to the pre-filled syringe by gently attaching the needle cap to the LLA adapter and turning it a quarter turn in the direction of the arrow, until you feel the needle click (as shown in figure 2).
- 3. You should remove the needle shield, which may offer some resistance.
- 4. You should add the solvent to the powder. Then, you should shake vigorously until the powder is completely dissolved.
- 5. You should withdraw the entire contents of the vial.
- 6. To administer the vaccine, you should use a new needle. To do this, you should unscrew the needle from the pre-filled syringe and attach the injection needle, following the instructions in point 2.
- 7.
After reconstitution, the vaccine should be used immediately or stored in a refrigerator (2°C - 8°C). If it is not used within 24 hours, it should be discarded.
Any unused medicinal product or waste material should be disposed of in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGlaxoSmithKline Biologicals S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Priorix-tetraDosage form: Powder, -Active substance: measles, combinations with mumps, rubella and varicella, live attenuatedManufacturer: GlaxoSmithKline Biologicals S.A.Prescription requiredDosage form: Powder, -Active substance: measles, combinations with mumps and rubella, live attenuatedManufacturer: GlaxoSmithKline Biologicals S.A.Prescription requiredDosage form: Suspension, 160 ELISA antigen units of hepatitis A virus, strain GBM/0.5 ml; 1 dose (0.5 ml)Active substance: hepatitis A, inactivated, whole virusPrescription required
Alternatives to Priorix-tetra in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Priorix-tetra in Spain
Alternative to Priorix-tetra in Ukraine
Online doctors for Priorix-tetra
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Priorix-tetra – subject to medical assessment and local rules.














