
PRIORIX, POWDER AND SOLVENT FOR INJECTION IN PRE-FILLED SYRINGE

How to use PRIORIX, POWDER AND SOLVENT FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Priorix is and what it is used for
- What you need to know before you are given Priorix
- How Priorix is administered
- Possible side effects
- Storage of Priorix
- Contents of the pack and further information
- The detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- -----------------------------------------------------------------------------------------------------------------------------
- This information is intended only for healthcare professionals:
Introduction
Package Leaflet: Information for the User
Priorix, powder and solvent for solution for injection in a pre-filled syringe
Vaccine against measles, mumps and rubella (live)
Read all of this leaflet carefully before you are given this vaccine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed for you, do not pass it on to others.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
This leaflet has been written assuming that the person reading it is the one receiving the vaccine, but it may be administered to adults and children, so you may be reading it for your child.
Contents of the pack and other information
- What Priorix is and what it is used for
- What you need to know before you are given Priorix
- How Priorix is administered
- Possible side effects
- Storage of Priorix
Contents of the pack and further information
1. What Priorix is and what it is used for
Priorix is a vaccine that is administered to children from 9 months, adolescents and adults to protect them against diseases caused by the measles, mumps and rubella viruses.
How Priorix works
When a person is vaccinated with Priorix, the immune system (the body's natural defense system) will produce antibodies to protect the person from infection with the measles, mumps and rubella viruses.
Although Priorix contains live viruses, they are too weak to cause measles, mumps or rubella in healthy people.
2. What you need to know before you are given Priorix
Priorix must not be administered if
- you are allergic to the active substances or to any of the other components of this vaccine (listed in section 6). The signs of an allergic reaction may include skin rash with itching, difficulty breathing and swelling of the face or tongue;
- you are allergic to neomycin (an antibiotic). Although a known contact dermatitis (skin rash caused by direct contact with allergens such as neomycin) should not be a problem, consult your doctor first;
- you have a severe infection with a high temperature. In these cases, vaccination will be postponed until you recover. Although a mild infection, such as a cold, should not be a problem, consult your doctor first;
- you have a disease (such as Human Immunodeficiency Virus (HIV) or Acquired Immune Deficiency Syndrome (AIDS)) or are taking any medication that may weaken the immune system. Whether or not you receive the vaccination will depend on the level of your defenses;
- you are pregnant. Additionally, pregnancy should be avoided for 1 month after vaccination.
Warnings and precautions
Consult your doctor or pharmacist before you are vaccinated with Priorix if:
- you have central nervous system disorders, a history of seizures accompanied by high fever or a family history of seizures. In case of high fever after vaccination, consult your doctor immediately;
- you have ever had a severe allergic reaction to egg proteins;
- you have had an adverse reaction after vaccination against measles, mumps or rubella that involved easy bruising or bleeding for longer than usual (see section 4);
- you have a weakened immune system (e.g., HIV infection). You should be closely monitored as the response to vaccination may not be sufficient to ensure protection against the disease (see section 2 "Priorix must not be administered if").
Before or after any injection, fainting (especially in adolescents) may occur, so you should inform your doctor or nurse if you have fainted after receiving an injection in the past.
If you are vaccinated within 72 hours of contact with someone who has measles, Priorix will provide some protection against the disease.
Children under 12 months
It is possible that children vaccinated during their first year of life may not be fully protected. Your doctor will advise you if additional doses of the vaccine are required.
As with all vaccines, it is possible that Priorix may not fully protect all vaccinated individuals.
Use of Priorix with other medicines
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines (or other vaccines).
Priorix can be administered to you at the same time as other vaccines such as diphtheria, tetanus, pertussis, Haemophilus influenzaetype b, oral or inactivated polio, hepatitis A, hepatitis B, varicella, meningococcal group B vaccines, as well as meningococcal group C, meningococcal groups A, C, W-135, Y and pneumococcal conjugate vaccine. Consult your doctor or nurse for more information.
A different injection site should be used for each vaccine.
If they are not administered at the same time, an interval of at least one month is recommended between the administration of Priorix and other live attenuated vaccines.
Your doctor may delay vaccination for at least 3 months if you have received blood transfusions or human antibodies (immunoglobulins).
If a tuberculin test is to be performed, it should be done before, at the same time as, or 6 weeks after vaccination with Priorix.
Pregnancy, breastfeeding and fertility
Priorix must not be administered to pregnant women.
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before vaccination. It is also important that you do not become pregnant for 1 month after vaccination. During this time, you should use an effective method of contraception to prevent pregnancy.
In the event of unintended vaccination of pregnant women with Priorix, this should not be a reason to terminate the pregnancy.
Priorix contains sorbitol, para-aminobenzoic acid, phenylalanine, sodium and potassium
This vaccine contains 9 mg of sorbitol per dose.
Priorix contains para-aminobenzoic acid. It may cause allergic reactions (possibly delayed) and, exceptionally, bronchospasm.
This vaccine contains 334 micrograms of phenylalanine per dose. Phenylalanine may be harmful in cases of phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body is unable to eliminate it properly.
This vaccine contains less than 23 mg of sodium (1 mmol) per dose; it is essentially "sodium-free".
This vaccine contains less than 39 mg (1 mmol) of potassium per dose, so it is considered essentially "potassium-free".
3. How Priorix is administered
Priorix is administered under the skin or into the muscle, either in the upper arm or on the outer thigh.
Priorix is intended for children from 9 months, adolescents and adults.
Your doctor will determine the timing and number of injections suitable for you based on official recommendations.
The vaccine should never be administered into a vein.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
The side effects that occurred in clinical trials with Priorix were:
- Very common (may occur in more than 1 in 10 doses of the vaccine):
- redness at the injection site
- fever of 38°C or higher.
- Common (may occur in up to 1 in 10 doses of the vaccine):
- pain and swelling at the injection site
- fever above 39.5°C
- skin rash (spots)
- upper respiratory tract infection.
- Uncommon (may occur in up to 1 in 100 doses of the vaccine):
- middle ear infection
- swelling of the lymph nodes (nodes in the neck, armpit or groin)
- loss of appetite
- nervousness
- abnormal crying
- inability to sleep (insomnia)
- red, irritated and watery eyes (conjunctivitis)
- bronchitis
- cough
- swelling of the parotid glands (glands in the cheeks)
- diarrhea
- vomiting.
- Rare (may occur in up to 1 in 1,000 doses of the vaccine):
- seizures accompanied by high fever
- allergic reactions.
After Priorix has been marketed, the following side effects have been reported:
- joint and muscle pain
- appearance of small spots or patches on the skin or bruising more easily than usual due to a decrease in the number of platelets
- sudden life-threatening allergic reaction
- infection or inflammation of the brain, spinal cord and peripheral nerves causing temporary difficulty walking (unsteadiness) and/or temporary loss of control of body movements, inflammation of some nerves, possibly with tingling or loss of normal sensation or movement (Guillain-Barré syndrome)
- narrowing or blockage of blood vessels
- erythema multiforme (symptoms are red patches, often accompanied by itching, similar to the skin rash caused by measles, starting on the limbs and sometimes on the face and the rest of the body)
- symptoms similar to those of measles and mumps (including painful and temporary swelling of the testicles and swelling of the glands in the neck)
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use, www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Priorix
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the carton, after EXP.
Store and transport refrigerated (between 2°C and 8°C).
Do not freeze.
Store in the original packaging to protect it from light.
The vaccine should be administered immediately after reconstitution. If this is not possible, it should be stored in the refrigerator (between 2°C and 8°C) and used before 8 hours after reconstitution.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicines in the pharmacy's SIGRE collection point. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Priorix composition
- The active substances are: live attenuated measles, mumps and rubella viruses.
- The other components are:
Powder: amino acids (containing phenylalanine), lactose (anhydrous), mannitol (E-421), sorbitol (E-420), medium 199 (containing phenylalanine, para-aminobenzoic acid, sodium and potassium).
Solvent: water for injections.
Appearance of the product and pack contents
Priorix is presented as a powder and solvent for solution for injection (powder in a vial for 1 dose and solvent in a pre-filled syringe (0.5 ml)), with or without needles, in the following pack sizes:
- with 2 separate needles: packs of 1 or 10
- without needle: packs of 1 or 10.
Priorix is supplied as a white to slightly pink powder, some of which may be yellowish to slightly orange, and a clear and colorless solvent (water for injections) to reconstitute the vaccine.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder:
GlaxoSmithKline, S.A.
PTM - C/ Severo Ochoa, 2
28760 Tres Cantos, Madrid
Telephone: 900 202 700
Fax: 91 807 03 10
e-mail: [email protected]
Manufacturer:
GlaxoSmithKline Biologicals S.A.
Rue de l'Institut 89; 1330 Rixensart
Belgium
Date of last revision of this leaflet:07/2025
The detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
-----------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
As with all injectable vaccines, appropriate medical treatment and supervision should be available to deal with the rare possibility of anaphylactic reactions following administration of the vaccine.
Since alcohol and other disinfectants can inactivate the live attenuated viruses of the vaccine, they should be allowed to evaporate from the skin before administration.
Priorix must not be administered intravascularly under any circumstances.
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
Before reconstitution or administration, the solvent and the reconstituted vaccine should be inspected visually for any foreign particles and/or changes in physical appearance. If any are observed, the solvent or the reconstituted vaccine should not be used.
The vaccine should be reconstituted by adding the entire contents of the pre-filled syringe to the vial containing the powder.
To learn how to insert the needle into the syringe, read the instructions provided with images 1 and 2 carefully. However, the syringe provided with Priorix may be slightly different (without screw thread) from the syringe in the image. In this case, the needle should be inserted without screwing.
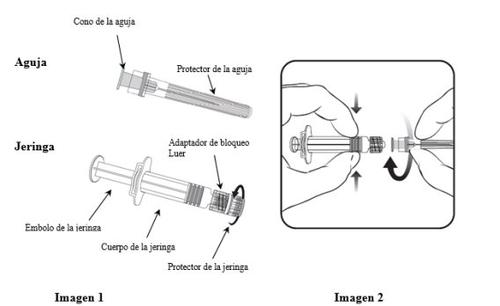
Always hold the syringe by the body, not by the plunger or the Luer adapter (LA), and keep the needle in line with the syringe (as shown in image 2). Otherwise, the LA could become deformed and cause leaks.
If the LA comes off during syringe assembly, use a new dose of the vaccine (new syringe and vial).
- Unscrew the syringe protector by turning it counterclockwise (as shown in image 1).
Whether or not the LA turns, please follow the next steps:
- Insert the needle into the syringe by gently fitting the cone of the needle into the LA and turning a quarter turn clockwise until it clicks (as shown in image 2).
- Remove the needle protector (it may be difficult).
- Add the solvent to the powder. Shake the mixture well until the powder is completely dissolved.
Due to minor variations in its pH, the color of the reconstituted vaccine may vary from light orange to pink without affecting the potency of the vaccine.
- Withdraw the entire contents of the vial.
- A new needle should be used to administer the vaccine. Unscrew the needle from the syringe and insert the needle for injection, repeating the previous step 2.
The vaccine should be administered immediately after reconstitution. If this is not possible, it should be stored between 2°C and 8°C and used before 8 hours after reconstitution.
Disposal of unused medicinal products and all materials that have come into contact with them should be done in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PRIORIX, POWDER AND SOLVENT FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLEActive substance: measles, combinations with mumps and rubella, live attenuatedManufacturer: Merck Sharp & Dohme B.V.Prescription requiredDosage form: INJECTABLE, 1 MILES DICT50 VIRUS / 0.01 MILES DICT50 VIRUS / 12.5 MILES DICT50 VIRUSActive substance: measles, combinations with mumps and rubella, live attenuatedManufacturer: Merck Sharp & Dohme B.V.Prescription requiredDosage form: INJECTABLE, 3 Infectious dose 50% in tissue culture / 3 Infectious dose 50% in tissue culture / 4.3 Infectious dose 50% in tissue culture / 3.99 FIP UActive substance: measles, combinations with mumps, rubella and varicella, live attenuatedManufacturer: Merck Sharp & Dohme B.V.Prescription required
Online doctors for PRIORIX, POWDER AND SOLVENT FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about PRIORIX, POWDER AND SOLVENT FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















