
Prilotekal

Ask a doctor about a prescription for Prilotekal

How to use Prilotekal
Package Leaflet: Information for the User
Prilotekal
20 mg/ml, solution for injection
Prylocaine hydrochloride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
Keep this package leaflet, you may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
If you experience any side effects, including those not listed in this package leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet:
- 1. What is Prilotekal and what is it used for
- 2. Important information before using Prilotekal
- 3. How to use Prilotekal
- 4. Possible side effects
- 5. How to store Prilotekal
- 6. Contents of the pack and other information
1. What is Prilotekal and what is it used for
Prilotekal 20 mg/ml solution for injection is a type of medicine called a local anesthetic, belonging to the amide group, and is a solution for injection. Prilotekal solution for injection is used for anesthesia of certain parts of the body and to prevent pain during surgery in adults.
Prilotekal is injected into the lower spine of the patient. It helps to quickly stop pain in the patient from the waist down for a limited time (short-term surgical procedures).
2. Important information before using Prilotekal
When not to use Prilotekal
if you are allergic (hypersensitive) to prilocaine hydrochloride, other local anesthetics of the amide group, or any of the other ingredients of this medicine (listed in section 6), if you have severe heart conduction problems, if you have acute anemia, if you have uncontrolled heart failure, if you have hypovolemic or cardiogenic shock, if you have congenital or acquired methemoglobinemia, if you have general or specific contraindications to spinal anesthesia technique. Prilotekal should not be administered into a blood vessel. Prilotekal should not be used in children under 6 months of age.
Warnings and precautions
In case of any of the following conditions, inform your doctor before using this medicine.
if you have ever had an undesirable reaction to a local anesthetic
if you have a skin infection at the proposed injection site or nearby
if you suffer from any of the following conditions:
- diseases of the central nervous system, such as: meningitis, polio, and spinal cord problems due to anemia,
- severe headache,
- brain, spinal cord, or other tumors,
- spinal cord tuberculosis,
- recent spinal cord injury,
- very low blood pressure or low blood volume,
- blood clotting problems,
- acute porphyria,
- fluid in the lungs,
- sepsis (blood poisoning). if you have heart disease (including complete or partial heart block, uncontrolled heart failure, arrhythmia) if you have liver or kidney problems if you have a neurological disorder, such as multiple sclerosis, hemiplegia, transverse myelitis, or neuromuscular disorders if you have general weakness. Spinal anesthesia should only be administered by a doctor with the necessary knowledge and experience. The doctor is responsible for taking the necessary actions to avoid injecting the medicine into a blood vessel and for having the skills to recognize and treat side effects.
Children and adolescents
Prilotekal is not recommended for use in children and adolescents. The efficacy and safety of Prilotekal in children and adolescents have not been established. Data in this regard are not available.
The use of Prilotekal in children under 6 months of age is contraindicated due to the higher risk of developing methemoglobinemia.
Prilotekal and other medicines
Tell your doctor about all medicines you are taking or have recently taken, as well as any medicines you plan to take, including those that are available without a prescription.
Especially when taking medicines used to treat irregular heart rhythm (antiarrhythmic drugs of class III) and painkillers.
Pregnancy, breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor if you can use Prilotekal.
Do not administer prilocaine for local or regional anesthesia during childbirth.
It is not known whether prilocaine passes into breast milk. Breastfeeding can be resumed after 24 hours from the administration of the medicine.
Driving and using machines
Do not drive or operate any tools or machines, as Prilotekal may affect your reactions and muscle coordination for some time.
Prilotekal contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per dose (maximum dose corresponding to 4 ml of Prilotekal solution for injection), which means that the medicine is considered "sodium-free".
3. How to use Prilotekal
This medicine is administered to you by a doctor who will determine the correct dose for you. The usual dose for adults is 40-60 mg of prilocaine hydrochloride (2-3 ml of Prilotekal); the maximum dose is 80 mg of prilocaine hydrochloride (4 ml of Prilotekal).
The doctor will inject Prilotekal into your lower spine, and you will be in a sitting or lying position.
Prilotekal is not recommended for use in children and adolescents. The safety and efficacy of Prilotekal in children and adolescents have not been established. The use of Prilotekal in children under 6 months of age is contraindicated due to the higher risk of developing methemoglobinemia.
In patients with poor general condition and concomitant diseases (e.g., vascular occlusion, atherosclerosis, diabetic polyneuropathy, and poor liver or kidney function), a reduced dose is recommended.
In case of impaired kidney or liver function, lower doses are recommended.
Prilotekal is administered intrathecally.
Equipment, medicines, and personnel able to act in an emergency must be immediately available. In rare cases, after the administration of local anesthetics, severe reactions have been reported, even in the absence of hypersensitivity in the individual patient's history.
Overdose of Prilotekal
It is unlikely that you will receive too much Prilotekal if it is administered by a doctor experienced in spinal anesthesia. However, if the dose is accidentally injected directly into the bloodstream, the patient may develop problems with vision or hearing, muscle twitching, trembling, seizures, convulsions, and loss of consciousness within a short time. In any case, when you receive Prilotekal, appropriate equipment for treatment must be available in case of overdose.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Prilotekal can cause side effects, although not everybody gets them.
As with all local anesthetics, decreased blood pressure and slower heart rate may occur frequently.
You may feel nausea, have low blood pressure, or a slow heart rate. Other possible side effects include: post-operative headache, vomiting, and difficulty urinating.
Possible side effects are:
Very common: may affect more than 1 in 10 people
Low blood pressure, nausea.
Common: may affect up to 1 in 10 people
Paresthesia, dizziness, vomiting.
Uncommon: may affect up to 1 in 100 people
Seizures, paresthesia around the mouth, loss of consciousness, trembling, feeling of numbness of the tongue, speech problems, hearing problems, tinnitus, vision problems, back pain, transient muscle weakness. Slow heart rate, increased blood pressure.
Rare: may affect up to 1 in 1,000 people
Methemoglobinemia, cyanosis, anaphylactic shock, anaphylactic reactions, allergic reactions, itching.
Arachnoiditis, neuropathy, peripheral nerve changes.
Double vision. Cardiac arrest, irregular heartbeat. Respiratory depression.
It is unlikely that Prilotekal solution for injection will cause severe side effects, unless it is accidentally administered incorrectly or used at the same time as other local anesthetics. In such a case, numbness of the tongue, dizziness, dizziness, trembling, and seizures may occur. In very rare cases, after the administration of prilocaine, the following may occur: myocardial infarction, breathing problems, loss of sensation in the lower parts of the body, and allergic reactions, which can cause rash, swelling, or very low blood pressure.
A rare but serious side effect of spinal anesthesia is high or total spinal block, with subsequent depression of cardiovascular and respiratory function.
Reporting side effects
If you experience any side effects, including those not listed in this package leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Prilotekal
Keep the medicine out of the sight and reach of children.
Do not use Prilotekal after the expiry date stated on the ampoules and outer packaging. The expiry date (EXP) is the last day of the given month.
Do not store above 25°C. Do not refrigerate or freeze.
Store in the original packaging to protect from light.
Use immediately after opening.
Do not use Prilotekal if you notice that the solution is not clear and free of particles.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements. Due to the restriction on use in hospitals, the disposal of leftover medicine is performed directly by the hospital. This will help protect the environment.
6. Contents of the pack and other information
What Prilotekal contains
The active substance is prilocaine hydrochloride.
1 ml of solution for injection contains 20 mg of prilocaine hydrochloride (which corresponds to 2%).
1 ampoule with 5 ml of solution contains 100 mg of prilocaine hydrochloride.
The other ingredients are: anhydrous glucose or glucose monohydrate, water for injections, sodium hydroxide 1N (for pH adjustment).
What Prilotekal looks like and contents of the pack
Solution for injection. Clear, colorless solution.
Prilotekal is provided in ampoules made of colorless glass type I.
Box of 10 ampoules, each containing 5 ml of solution for injection.
Marketing authorization holder
B.Braun Melsungen AG
Carl-Braun-Strasse 1
34212 Melsungen
Germany
Manufacturer:
Sirton Pharmaceuticals SPA
Piazza XX Settembre 2
22079 Villa Guardia (CO)
Italy
Sintetica GmbH
Albersloher Weg 11
- 48155 - Münster Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
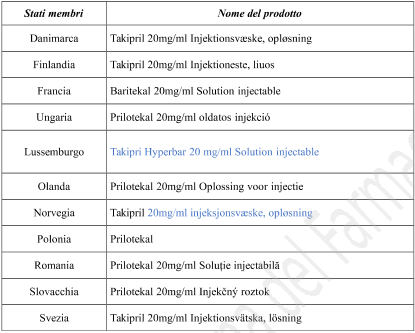
| Member State | Medicinal product name |
| Austria | Takipril hyperbar 2% Injektionslösung |
| Germany | Takipril 20 mg/ml Injektionslösung |
| Italy | Prilotekal |
| Spain | Takipril hiperbárica, 20mg/ml solución inyectable |
| United Kingdom | Prilotekal 20mg/ml solution for injection |
| Belgium | Tachipri Hyperbar 20 mg/ml Oplossing voor injectie Tachipri Hyperbar 20 mg/ml Solution injectable Tachipri Hyperbar 20 mg/ml Injektionslösung |
| Bulgaria | Takipril 20 mg/ml Инжекционен разтвор |
| Czech Republic | Takiprin |
| Denmark | Takipril 20 mg/ml Injektionsvæske, opløsning |
| Finland | Takipril 20 mg/ml Injektioneste, liuos |
| France | Baritekal 20 mg/ml Solution injectable |
| Hungary | Prilotekal 20 mg/ml oldatos injekció |
| Luxembourg | Tachipri Hyperbar 20 mg/ml Solution injectable |
| Netherlands | Prilotekal 20 mg/ml Oplossing voor injectie |
| Norway | Takipril 20 mg/ml injeksjonsvæske, oppløsning |
| Poland | Prilotekal |
| Romania | Prilotekal 20 mg/ml Soluţie injectabilă |
| Slovakia | Prilotekal 20 mg/ml Injekčný roztok |
| Sweden | Takipril 20 mg/ml Injektionsvätska, lösning |
Date of last revision of the package leaflet: September 2020
The following information is intended for healthcare professionals only:
{ The SmPC is attached at the end of the printed package leaflet, as a detachable part.}
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterSintetica GmbH Sirton Pharmaceuticals SpA.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PrilotekalDosage form: Cream, (25 mg + 25 mg)/gActive substance: lidocaine, combinationsManufacturer: Pierre Fabre Medicament ProductionPrescription requiredDosage form: Gel, 0.5 g/100 gActive substance: lidocainePrescription not requiredDosage form: Aerosol, 96 mg/gActive substance: lidocaineManufacturer: Aflofarm Farmacja Polska Sp. z o.o.Prescription not required
Alternatives to Prilotekal in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Prilotekal in Spain
Online doctors for Prilotekal
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Prilotekal – subject to medical assessment and local rules.














