
Bravera Control

Ask a doctor about a prescription for Bravera Control

How to use Bravera Control
Package Leaflet: Information for the Patient
Bravera Control
96 mg/g, spray for the skin, solution
Lidocaine
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient or as advised by a doctor or pharmacist.
- This leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, the doctor or pharmacist should be informed. See section 4.
- If there is no improvement or the patient feels worse, a doctor should be contacted.
Table of Contents of the Leaflet
- 1. What is Bravera Control and what is it used for
- 2. Important information before using Bravera Control
- 3. How to use Bravera Control
- 4. Possible side effects
- 5. How to store Bravera Control
- 6. Contents of the pack and other information
1. What is Bravera Control and what is it used for
Bravera Control contains lidocaine. Lidocaine acts as a local anesthetic.
The anesthetic effect of the medicine is to reduce the sensitivity of the glans penis, in order to prolong
the time to ejaculation.
The medicine is indicated for use before intercourse in the case of premature ejaculation, in order to
reduce the sensitivity of the penis to touch and delay ejaculation (ejaculation).
If there is no improvement or the patient feels worse, a doctor should be consulted.
2. Important information before using Bravera Control
When not to use Bravera Control:
- if the patient or their partner has an allergy to lidocaine or any of the other ingredients of this medicine (listed in section 6);
- if the patient or their partner has an allergy to other amide local anesthetics;
- if the patient's partner is pregnant;
- on damaged or inflamed skin.
Warnings and precautions
Before starting to use Bravera Control, the patient should discuss it with a doctor or pharmacist.
- If the patient has or has ever had liver or kidney problems, they should consult a doctor before using the medicine.
- If, during the use of the medicine, the patient or their partner experiences a rash or irritation, the use of the medicine should be stopped and a doctor consulted.
The medicine should not come into contact with the eyes and mucous membranes of the patient's or their partner's mouth, nose, and throat.
In case of contact with the eyes, they should be rinsed immediately with cold water.
During use, the medicine may also come into contact with the mucous membranes of other areas of the body,
such as the mouth, nose, and throat of the patient or their partner, causing temporary numbness. Since the effect of the medicine reduces the ability to feel pain in these areas, special care should be taken until the numbness subsides, to avoid injuring these areas.
During intercourse, a small amount of the medicine may be transferred, for example, to the vagina or anus.
As a result, partners may temporarily experience numbness and should be careful not to cause injury, especially during sexual activities.
Children and adolescents
The medicine should not be used in children and adolescents under 18 years of age.
Bravera Control and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Interactions may occur with:
- cardiac medicines (anti-arrhythmics);
- antiepileptic medicines;
- anticholinergic medicines (e.g., used to relieve stomach cramps, treat Parkinson's disease, or prevent motion sickness);
- antihypertensive medicines;
- barbiturates (e.g., sleeping pills or used to treat epilepsy);
- beta-blockers (medicines used to treat high blood pressure and regulate heart rhythm);
- muscle relaxants;
- sympathomimetic medicines (e.g., used to treat heart failure, asthma, or colds). It has also been reported to affect diagnostic tests for serum enzymes.
Pregnancy and breastfeeding
If the patient's partner is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
The medicine is not indicated for use in women.
The medicine should not be used if the patient's partner is pregnant.
Driving and using machines
The medicine has no effect on the ability to drive and use machines.
3. How to use Bravera Control
This medicine should always be used exactly as described in the package leaflet for the patient, or as advised by a doctor or pharmacist. If in doubt, the patient should consult a doctor or pharmacist.
Method of administration
Administration on the skin (locally on the shaft and glans penis).
Adults
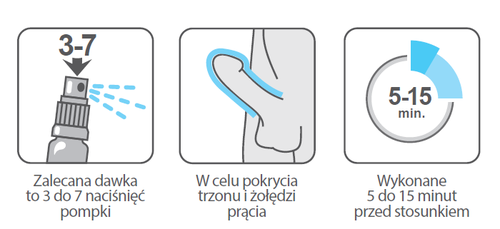
The recommended dose is 3 to 7 sprays, applied to cover the shaft and glans penis, 5 to 15 minutes before intercourse.
If, after 5 minutes, there is an excess of the medicine on the penis, it should be removed before intercourse.
The amount and time of application should be adjusted according to individual needs.
The smallest effective dose should always be used.
There should be an interval of at least 4 hours between applications of the medicine.
The dose should not exceed 22 sprays in 24 hours.
The medicine should not be used for more than 1 month without medical supervision.
Instructions for using Bravera Control
Before the first use of the medicine, the patient should, while holding the container vertically, press the spray pump at least 10 times, to fill it and obtain the correct spray.
If the spray pump has not been used for at least 10 days, it should be prepared again by pressing it three times and spraying the medicine into the air.
The container should be directed away from the face, to avoid accidental contact of the medicine with the eyes, ears, nose, and mouth.
The foreskin should be retracted from the glans penis. Holding the container in a vertical position (with the valve upwards), the patient should apply 3 to 7 sprays to the entire surface of the glans penis and shaft, 5 to 15 minutes before intercourse.
If, after 5 minutes, there is an excess of the medicine on the penis, it should be removed before intercourse.
Use in children and adolescents
The medicine should not be used in children and adolescents under 18 years of age.
Elderly
The medicine is not recommended for use in the elderly.
Use of a higher than recommended dose of Bravera Control
Since the medicine is applied to the surface of the penis, the risk of overdose is low.
In the event of an overdose, skin irritation may occur at the site of application.
In the event of an overdose, the excess medicine should be removed and a doctor consulted.
If there are any further doubts about the use of this medicine, the patient should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Bravera Control can cause side effects, although not everybody gets them.
After using the medicine, local skin irritation or rash may very rarely occur.
In such cases, the use of the medicine should be stopped.
No other side effects have been reported after administration of the recommended doses of the medicine.
If higher doses are used or there is hypersensitivity to lidocaine, the following side effects may occur:
Very rare(occurring in less than 1 in 10,000 people):
- symptoms of central nervous system disorders, excitement, nervousness;
- symptoms of central nervous system depression. Dizziness, drowsiness, convulsions, loss of consciousness.
Frequency not known(cannot be estimated from the available data):
Weakness of the heart muscle, bradycardia (slow heart rate), cardiac arrest, hypotension, respiratory failure.
Reporting side effects
If any side effects occur, including any possible side effects not listed in the leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Bravera Control
The medicine should be stored out of sight and reach of children.
Store in a temperature below 25°C. Store in the original packaging to protect from light.
Do not use this medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of the month.
Shelf life after first opening: 3 months.
Medicines should not be disposed of via wastewater or household waste. A pharmacist should be consulted on how to dispose of unused medicines. This will help protect the environment.
6. Contents of the pack and other information
What Bravera Control contains
- The active substance of the medicine is lidocaine. 1 g of spray contains 96 mg of lidocaine. Each spray of the pump delivers 0.085 ml of solution (which corresponds to 81.98 mg of spray containing 7.87 mg of lidocaine).
- The other ingredients are: stearyl acid, isopropyl myristate, diethylene glycol monoethyl ether.
What Bravera Control looks like and contents of the pack
Bravera Control, 96 mg/g, spray for the skin, solution, is a colorless to yellow solution.
The packaging of the medicine is a HDPE bottle with a spray pump, consisting of polypropylene (PP), polyethylene (PE), polyoxymethylene (POM), and steel, containing 8 ml of solution, in a cardboard box with a leaflet.
Marketing authorization holder and manufacturer
Aflofarm Farmacja Polska Sp. z o.o.
Partyzancka 133/151
95-200 Pabianice
phone: (42) 22-53-100
Date of last revision of the leaflet:28.09.2022
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAflofarm Farmacja Polska Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Bravera ControlDosage form: Gel, 0.5 g/100 gActive substance: lidocainePrescription not requiredDosage form: Gel, 20 mg/gActive substance: lidocaineManufacturer: Chemische Fabrik Kreussler Co.GmbHPrescription not requiredDosage form: Gel, 20 mg/mlActive substance: lidocaineManufacturer: Klosterfrau Berlin GmbHPrescription not required
Alternatives to Bravera Control in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Bravera Control in Spain
Alternative to Bravera Control in Ukraine
Online doctors for Bravera Control
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Bravera Control – subject to medical assessment and local rules.














