

Polopirina Gardvo Sprai

Ask a doctor about a prescription for Polopirina Gardvo Sprai

How to use Polopirina Gardvo Sprai
Package Leaflet: Information for the User
POLOPIRYNA GARDŁO SPRAY, 8.75 mg/dose, oral spray, solution
Flurbiprofen
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, consult a pharmacist.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 3 days there is no improvement or you feel worse, consult a doctor.
Table of Contents of the Leaflet
- 1. What is Polopiryna Gardło Spray and what is it used for
- 2. Important information before using Polopiryna Gardło Spray
- 3. How to use Polopiryna Gardło Spray
- 4. Possible side effects
- 5. How to store Polopiryna Gardło Spray
- 6. Contents of the pack and other information
1. What is Polopiryna Gardło Spray and what is it used for
The active substance is flurbiprofen. Flurbiprofen belongs to a group of medicines called nonsteroidal anti-inflammatory drugs (NSAIDs), which work by changing the body's response to pain, swelling, and high temperature.
Polopiryna Gardło Spray is intended for short-term relief of symptoms of inflammatory throat conditions, such as irritation, sore throat, difficulty swallowing, and swelling in adults aged 18 and over.
2. Important information before using Polopiryna Gardło Spray
When not to use Polopiryna Gardło Spray
- If you are allergic to flurbiprofen, other nonsteroidal anti-inflammatory drugs (NSAIDs), acetylsalicylic acid, or any of the other ingredients of this medicine (listed in section 6).
- if you have had an allergic reaction in the past after using nonsteroidal anti-inflammatory drugs (NSAIDs) or acetylsalicylic acid, such as asthma, wheezing, itching, rhinitis, skin rash, swelling;
- if you have had two or more episodes of stomach ulcers, bleeding, or intestinal ulcers;
- if you have had severe colitis (inflammation of the large intestine);
- if you have had coagulation disorders or bleeding problems after taking NSAIDs;
- if you are in the last 3 months of pregnancy;
- if you have severe heart, kidney, or liver failure.
Warnings and precautions
Before starting to use Polopiryna Gardło Spray, discuss it with your doctor or pharmacist:
- if you are already taking other nonsteroidal anti-inflammatory drugs (NSAIDs) or acetylsalicylic acid;
- if you have tonsillitis or suspect you may have a bacterial throat infection (you may need an antibiotic);
- if you are elderly (you may be more prone to side effects);
- if you have or have had asthma or suffer from allergies;
- if you have a skin disease called systemic lupus erythematosus or mixed connective tissue disease;
- if you have high blood pressure;
- if you have had intestinal disease (ulcerative colitis, Crohn's disease);
- if you have heart, kidney, or liver disease;
- if you have had a stroke;
- if you are breastfeeding or plan to become pregnant,
- if you have an infection - see "Infections" below.
During the use of Polopiryna Gardło Spray
- At the first signs of any skin reactions (rash, peeling, blisters) or the occurrence of other symptoms of an allergic reaction, discontinue the use of the spray and consult a doctor.
- Report any unusual abdominal symptoms (especially bleeding) to your doctor.
- If there is no improvement, or you feel worse or new symptoms appear, consult a doctor.
- Medicines like flurbiprofen may slightly increase the risk of heart attack (myocardial infarction) or stroke. The risk is more likely when using higher doses or long-term treatment. Do not exceed the recommended dose or duration of treatment (see section 3).
Infections
Nonsteroidal anti-inflammatory drugs (NSAIDs) may mask the symptoms of infection, such as fever and pain. This may delay proper treatment of infections, which can lead to increased risk of complications. If you are taking this medicine during an ongoing infection and your symptoms persist or worsen, consult your doctor or pharmacist immediately.
Children and adolescents
The medicine should not be used in children and adolescents under 18 years of age.
Polopiryna Gardło Spray and other medicines
Tell your doctor or pharmacist about all the medicines you are taking now or have taken recently, as well as any medicines you plan to take, including those available without a prescription. In particular, about:
- other nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 inhibitors used to treat pain or inflammation, as they may increase the risk of stomach or intestinal bleeding;
- warfarin, acetylsalicylic acid, and other blood-thinning medicines;
- angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists (blood pressure-lowering medicines);
- diuretics, including potassium-sparing diuretics;
- selective serotonin reuptake inhibitors (SSRIs) used to treat depression;
- digitalis glycosides (medicines used for heart conditions), such as digoxin;
- cyclosporine (prevents organ rejection after transplantation);
- corticosteroids (reduce inflammation);
- lithium (treats mood disorders);
- methotrexate (treats psoriasis, arthritis, and cancer);
- mifepristone (used for abortion): do not use NSAIDs during the 8-12 days after mifepristone administration, as they may reduce the effectiveness of mifepristone;
- oral antidiabetic medicines;
- phenytoin (treats epilepsy);
- probenecid, sulfinpyrazone (treats gout and arthritis);
- quinolone antibiotics (treats bacterial infections), such as ciprofloxacin, levofloxacin;
- tacrolimus (immunosuppressive medicine used after organ transplantation);
- zydovudine (treats HIV infection).
Polopiryna Gardło Spray with food, drink, and alcohol
While using this medicine, avoid consuming alcohol, as it may increase the risk of stomach or intestinal bleeding.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
Oral forms (e.g., tablets) of flurbiprofen may cause side effects in the unborn child. It is not known if the same risk applies to Polopiryna Gardło Spray.
Pregnancy
Do not use the medicine in the last trimester of pregnancy.
Do not use Polopiryna Gardło Spray during the first 6 months of pregnancy unless absolutely necessary and recommended by a doctor. If treatment is necessary during this period, use the smallest dose for the shortest possible time.
Breastfeeding
Do not use the medicine while breastfeeding.
Fertility
Flurbiprofen belongs to a group of medicines that may impair fertility in women. This effect is reversible after stopping the medicine. It is unlikely that occasional use of this medicine will affect the chances of becoming pregnant; however, tell your doctor before using the medicine if you have problems becoming pregnant.
Driving and using machines
This medicine should not affect your ability to drive or use machines.
However, after taking nonsteroidal anti-inflammatory drugs (NSAIDs), dizziness and visual disturbances may occur. In such cases, do not drive or operate machinery.
Polopiryna Gardło Spray contains cyclodextrins (betadex and hydroxypropylbetadex)
The medicine contains 9.91 mg of cyclodextrin (9.58 mg of betadex and 0.33 mg of hydroxypropylbetadex) in each dose (3 sprays), which corresponds to 19.15 mg/ml of cyclodextrin (18.50 mg/ml of betadex and 0.65 mg/ml of hydroxypropylbetadex).
3. How to use Polopiryna Gardło Spray
This medicine should always be used exactly as described in the package leaflet or as directed by a doctor or pharmacist. If you are unsure, consult your doctor or pharmacist.
Recommended dose
Adults over 18 years:One dose (3 sprays) to the back of the throat every 3-6 hours as needed. Do not take more than 5 doses in 24 hours.
One dose (3 sprays) contains 8.75 mg of flurbiprofen.
Use in children and adolescents
Do not use the medicine in children and adolescents under 18 years of age.
For oral use only.
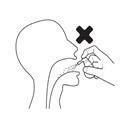
- Spray the aerosol only to the back of the throat.
- Do not inhale while spraying the aerosol.
- Do not take more than 5 doses (15 sprays) in 24 hours.
Polopiryna Gardło Spray is intended for short-term use only.
Use the smallest effective dose for the shortest duration necessary to relieve symptoms. If you have an infection, consult your doctor or pharmacist immediately if your symptoms (such as fever and pain) persist or worsen (see section 2).
Do not use the medicine for more than 3 days unless advised by a doctor.
If there is no improvement, or you feel worse or new symptoms appear, consult a doctor or pharmacist.
Preparing the pump
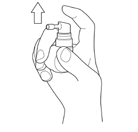
Before first use (or after storage for a longer period), shake the bottle and then activate the pump.
Direct the nozzle away from yourself and spray the aerosol at least four times until a uniform, light mist is obtained. The pump is activated and ready for use. If the medicine is not used for some time, direct the nozzle away from yourself and spray the aerosol at least once to obtain a uniform, light mist. Before using the medicine, always make sure a uniform, light mist is produced.
Using the aerosol
Direct the nozzle towards the back of the throat.
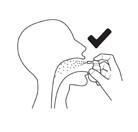
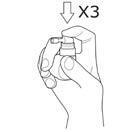
Press the pump three timeswith a quick, smooth motion, making sure to press the pump completely at each spray, removing your finger from the top of the pump between each spray.
Do not inhale while spraying the aerosol.
Using more than the recommended dose of Polopiryna Gardło Spray
Consult a doctor or pharmacist or go to the nearest hospital. Symptoms of overdose may include: nausea or vomiting, abdominal pain or less frequently diarrhea. There may be ringing in the ears, headache, and gastrointestinal bleeding.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them .
STOP TAKINGthe medicine and contact a doctor immediately if you experience any of the following symptoms:
- symptoms of an allergic reaction, such as asthma, unexplained wheezing or shortness of breath, itching, rhinitis, or skin rash;
- swelling of the face, tongue, or throat, causing difficulty breathing, palpitations, low blood pressure leading to shock (may occur even after the first use of the medicine);
- symptoms of hypersensitivity and skin reactions, such as redness, swelling, peeling, blistering, or ulceration of the skin and mucous membranes.
Other side effects may occur:
If you experience any of the following symptoms or side effects not listed in this leaflet, consult a doctor or pharmacist:
Common(may affect up to 1 in 10 people)
- dizziness, headache
- irritation of the throat
- mouth ulcers, pain, and numbness in the mouth
- sore throat
- discomfort (feeling of heat, burning, or tingling) in the mouth
- nausea and diarrhea
- tingling and itching of the skin
Uncommon(may affect up to 1 in 100 people)
- drowsiness
- blisters in the mouth or throat, feeling of numbness in the throat
- abdominal bloating, abdominal pain, gas, constipation, indigestion, vomiting
- dry mouth
- burning sensation in the mouth, taste disturbances
- skin rash, itching
- fever, pain
- drowsiness or difficulty sleeping
- worsening of asthma, wheezing, shortness of breath
- decreased sensation in the throat Rare(may affect up to 1 in 1,000 people)
- anaphylactic reaction
Frequency not known(cannot be estimated from the available data)
- anemia, thrombocytopenia (low platelet count, which can cause bruising and bleeding)
- edema, high blood pressure, heart failure, or heart attack
- severe skin reactions, such as blistering, including Stevens-Johnson syndrome and toxic epidermal necrolysis (rare diseases caused by severe side effects of the medicine or infection, in which there is a strong reaction of the mucous membranes and skin)
- hepatitis
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Polopiryna Gardło Spray
Store the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and bottle.
The expiry date refers to the last day of the month.
Shelf life after first opening: 1 month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Polopiryna Gardło Spray contains
The active substance is flurbiprofen. One dose (3 sprays) contains 8.75 mg of flurbiprofen.
One spray contains 2.92 mg of flurbiprofen. 1 ml of solution contains 17.16 mg of flurbiprofen.
The other ingredients are: sodium saccharin (E 954), citric acid, sodium hydroxide, disodium phosphate dodecahydrate, betadex (E 459), hydroxypropylbetadex, cherry flavor, purified water.
Composition of cherry flavor: flavoring and aroma ingredients, ethanol, glycerol triacetate, propylene glycol (E 1520), ascorbic acid, DL-alpha-tocopherol, purified water.
What Polopiryna Gardło Spray looks like and contents of the pack
Oral spray, solution is a clear, colorless solution with a cherry flavor and aroma.
Polopiryna Gardło Spray consists of a plastic bottle with a solution and a dosing device in the form of a mechanical pump for spraying.
Each bottle contains 15 ml of solution, sufficient for 88 sprays.
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA SA
ul. Pelplińska 19, 83-200 Starogard Gdański, Poland
phone: +48 22 364 61 01
Manufacturer
Bohm, S.A.
C/ Molinaseca 23
Poligono Industrial Cobo Calleja
28947 Fuenlabrada, Madrid
Spain
Laboratorium Sanitatis S.L.
C/ Leonardo Da Vinci, 11
(Parque Tecnológico de Álava)
01510 Miñano (Álava)
Spain
S.C. Terapia SA
Strada Fabricii nr. 124,
Cluj-Napoca 400632 Judet Cluj
Romania
Industria Quimica Y Farmaceutica Vir S.A.
C/ De La Laguna 66 68 70
Poligono Industrial Urtinsa II
28923 Alcorcon, Madrid
Spain
Date of last revision of the leaflet:October 2023
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterBohm S.A. Industria Quimica y Farmaceutica VIR S.A. Laboratorium Sanitatis S.L. S.C. Terapia S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Polopirina Gardvo SpraiDosage form: Lozenge, 8.75 mgActive substance: flurbiprofenManufacturer: Centrafarm Services B.V. Lozy's Pharmaceuticals Pierre Fabre Medicament Production STADA Arzneimittel AGPrescription not requiredDosage form: Lozenges, 8.75 mgActive substance: flurbiprofenPrescription not requiredDosage form: Aerosol, 8.75 mg/doseActive substance: flurbiprofenPrescription not required
Alternatives to Polopirina Gardvo Sprai in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Polopirina Gardvo Sprai in Spain
Alternative to Polopirina Gardvo Sprai in Ukraine
Online doctors for Polopirina Gardvo Sprai
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Polopirina Gardvo Sprai – subject to medical assessment and local rules.






