

Polcrom 2%

Ask a doctor about a prescription for Polcrom 2%

How to use Polcrom 2%
Leaflet attached to the packaging: patient information
POLCROM 2%, 20 mg/ml (2.8 mg/dose), nasal spray, solution
Sodium cromoglicate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as advised by a doctor or pharmacist.
- Keep this leaflet, so you can read it again if you need to.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor or pharmacist. See section 4.
- If there is no improvement or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Polcrom 2% and what is it used for
- 2. Important information before using Polcrom 2%
- 3. How to use Polcrom 2%
- 4. Possible side effects
- 5. How to store Polcrom 2%
- 6. Contents of the packaging and other information
1. What is Polcrom 2% and what is it used for
Polcrom 2% is an anti-allergic medicine containing sodium cromoglicate. It inhibits the release of histamine and other cellular mediators during allergic reactions. This prevents the occurrence of an allergic reaction.
The medicine is used preventively in the treatment of seasonal and year-round allergic rhinitis.
Use should be started before exposure to the allergen.
2. Important information before using Polcrom 2%
When not to use Polcrom 2%:
- if the patient is allergic to sodium cromoglicate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Polcrom 2%, the patient should discuss it with their doctor or pharmacist.
Children and adolescents
The medicine can be used in children over 3 years of age.
Polcrom 2% and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
No interactions between sodium cromoglicate and other nasal medicines have been observed.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
The medicine may be used during pregnancy only if necessary.
It is not known whether sodium cromoglicate passes into breast milk. However, caution should be exercised when using the medicine in breastfeeding women, although it is unlikely that the medicine will pass into breast milk and have adverse effects on the breastfed child.
Driving and using machines
The medicine does not affect the ability to drive or use machines.
Polcrom 2% contains benzalkonium chloride
The medicine contains 0.1 mg of benzalkonium chloride per ml of solution. Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time.
3. How to use Polcrom 2%
This medicine should always be used exactly as described in the patient leaflet or as advised by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
One dose contains 2.8 mg of sodium cromoglicate.
Adults, adolescents, and children over 3 years of age:
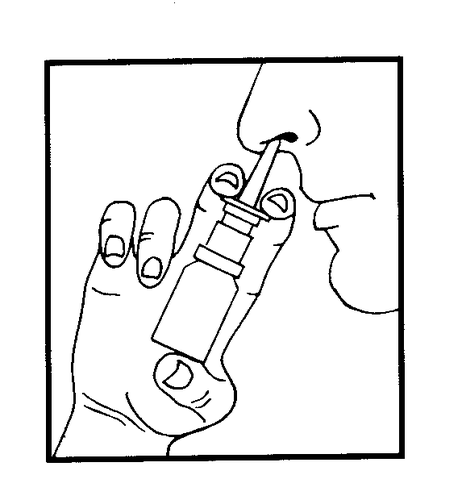
1 dose into each nostril 4 to 6 times a day.
Use of the medicine should be started at least one week before exposure to the allergen and continued throughout the exposure period.
Sodium cromoglicate is a preventive treatment. The medicine should be used regularly.
Method of administration
For hygiene reasons, the packaging of the medicine should only be used by one patient.
The medicine is for external use only - locally into the nose.
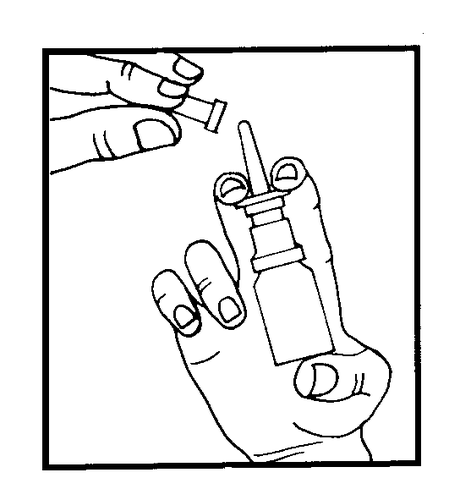
Each time before use, remove the protective cap.
Before using a new bottle for the first time, after removing the cap, press the dispenser 3-5 times until a mist of medicine appears. Insert the dispenser tip into the nostril. Holding the bottle upright, press the dispenser, which will spray a dose of medicine into the nose.
Using a higher dose of Polcrom 2% than recommended
No cases of sodium cromoglicate overdose or adverse effects of overdose have been reported, regardless of the route of administration.
Missing a dose of Polcrom 2%
If a dose of the medicine is missed, it should be taken as soon as possible. If it is almost time for the next dose, the missed dose should be skipped and the next dose taken as scheduled. A double dose should not be taken to make up for a missed dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Transient irritation of the nasal mucosa, nasal congestion, and swelling of the nasal mucosa, sneezing, and rare nosebleeds may occur.
Wheezing, feeling of tightness in the chest, coughing, taste disturbances, headache, rash, and hypersensitivity reactions, including severe anaphylactic reactions, may occur.
Reporting side effects
If any side effects occur, including any possible side effects not listed in this leaflet, the patient should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Polcrom 2%
Store at a temperature not exceeding 25°C.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Polcrom 2% contains
- The active substance is sodium cromoglicate. Each ml of solution contains 20 mg of sodium cromoglicate. One dose contains 2.8 mg of sodium cromoglicate.
- The other ingredients are: sorbitol, disodium phosphate dodecahydrate, sodium dihydrogen phosphate monohydrate, disodium edetate, sodium chloride, polysorbate 80, benzalkonium chloride solution, purified water.
What Polcrom 2% looks like and what the packaging contains
HDPE bottle with a metering pump in a cardboard box.
1 bottle of 15 ml
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
Medana branch in Sieradz
ul. Władysława Łokietka 10, 98-200 Sieradz
Date of last update of the leaflet:December 2024
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterZakłady Farmaceutyczne POLPHARMA S.A. Oddział Medana w Sieradzu
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Polcrom 2%Dosage form: Aerosol, 2.8 mg/nasal doseActive substance: cromoglicic acidManufacturer: Salutas Pharma GmbHPrescription not requiredDosage form: Aerosol, 1 mg/mlActive substance: azelastineManufacturer: Galenicum Health, S.L.U. SAG Manufacturing S.L.U.Prescription not requiredDosage form: Aerosol, 1.5 mg/mlActive substance: azelastinePrescription not required
Alternatives to Polcrom 2% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Polcrom 2% in Ukraine
Alternative to Polcrom 2% in Spain
Online doctors for Polcrom 2%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Polcrom 2% – subject to medical assessment and local rules.








