
Plizari
Ask a doctor about a prescription for Plizari

How to use Plizari
Leaflet accompanying the packaging: patient information
Plyzari, 6 mg/mL, solution for injection in a pre-filled pen
Liraglutide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Plyzari and what is it used for
- 2. Important information before using Plyzari
- 3. How to use Plyzari
- 4. Possible side effects
- 5. How to store Plyzari
- 6. Contents of the pack and other information
1. What is Plyzari and what is it used for
What is Plyzari
Plyzari is a weight-loss medicine that contains the active substance liraglutide. This substance is similar to a naturally occurring hormone, glucagon-like peptide-1 (GLP-1), which is released in the intestines after a meal. Plyzari works by affecting receptors in the brain that control appetite, causing a feeling of fullness and reducing the sensation of hunger. This can help limit the amount of food consumed and reduce body weight.
What is Plyzari used for
Plyzari is a weight-loss medicine used in combination with diet and exercise in adults aged 18 and over, who:
- Have a BMI of 30 kg/m² or higher (obesity) or
- Have a BMI of 27 kg/m² to less than 30 kg/m² (overweight) and have weight-related conditions (such as diabetes, high blood pressure, abnormal blood lipid levels, or sleep apnea). Body Mass Index (BMI) is a measure of body mass in relation to height.
Treatment with Plyzari should only be continued if, after 12 weeks of treatment with a dose of 3.0 mg/day, the initial body weight has decreased by at least 5% (see section 3). To continue treatment, consult a doctor. Plyzari may be used as an adjunct to a healthy diet and increased physical activity to control body weight in adolescents aged 12 and over with:
- obesity (diagnosed by a doctor)
- body weight over 60 kg
Treatment with Plyzari may be continued only if the body mass index has decreased by at least 4% after 12 weeks of treatment with a dose of 3.0 mg/day or the maximum tolerated dose (see section 3). To continue treatment, consult a doctor.
Diet and exercise
The doctor will recommend a diet and exercise program. Follow these recommendations during treatment with Plyzari.
2. Important information before using Plyzari
When not to use Plyzari
Warnings and precautions
Before starting treatment with Plyzari, discuss it with your doctor, pharmacist, or nurse. Treatment with Plyzari is not recommended for patients with severe heart failure. Experience with the use of Plyzari in patients aged 75 and over is limited. It is not recommended to use this medicine in patients aged 75 and over. There is limited data on the use of this medicine in patients with renal impairment. Patients with kidney dysfunction or patients on dialysis should consult a doctor. There is limited data on the use of this medicine in patients with liver failure. Patients with liver dysfunction should consult a doctor. It is not recommended to use this medicine in patients with severe stomach or intestinal disorders causing delayed gastric emptying (gastroparesis) or non-specific intestinal inflammation. If you are going to have surgery, tell your doctor that you are taking this medicine. Patients with diabetes Patients with diabetes should not use Plyzari as a substitute for insulin. Pancreatitis If you have or have had pancreatitis, consult a doctor. Gallbladder inflammation and gallstones During significant weight loss, the patient is at risk of developing gallstones and, consequently, gallbladder inflammation. Stop using Plyzari and consult a doctor immediately if you experience severe abdominal pain, usually stronger on the right side under the ribs. The pain may radiate to the back or right arm. See section 4.
Thyroid disease
In case of thyroid disease, including nodules and thyroid enlargement, consult a doctor.
Heart rate
If you experience palpitations (feeling of heartbeat) or a feeling of rapid heartbeat while at rest during treatment with Plyzari, consult a doctor.
Fluid loss and dehydration
After starting treatment with Plyzari, fluid loss or dehydration may occur. This may be caused by nausea (vomiting), vomiting, and diarrhea. Drink plenty of fluids to avoid dehydration. If you have any questions or concerns, consult a doctor, pharmacist, or nurse. See section 4.
Children and adolescents
The safety and efficacy of Plyzari in children under 12 years of age have not been studied.
Plyzari and other medicines
Tell your doctor, pharmacist, or nurse about all medicines you are taking or have recently taken, as well as any medicines you plan to take. In particular, inform your doctor, pharmacist, or nurse if:
- you are taking anti-diabetic medicines called sulfonylureas (such as glimepiride or glibenclamide) or insulin - low blood sugar (hypoglycemia) may occur when using Plyzari with these anti-diabetic medicines. Your doctor may need to adjust the dose of your anti-diabetic medicine to prevent low blood sugar. See section 4 for symptoms of low blood sugar.
- you are taking warfarin or other oral medicines that reduce blood clotting (anticoagulants). More frequent blood tests to check blood clotting may be necessary.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or plan to have a baby, do not use this medicine. There is no data on the effect of liraglutide on the health of the unborn child. Do not breastfeed during treatment with Plyzari. There is no information on the passage of liraglutide into breast milk.
Driving and using machines
Plyzari is unlikely to affect your ability to drive or use machines. However, some patients using this medicine may experience dizziness, mainly during the first 3 months of treatment (see "Possible side effects"). If you experience dizziness, be extra careful when driving or using machines. For more information, consult your doctor.
Plyzari contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which means it is essentially "sodium-free".
3. How to use Plyzari
Always use this medicine exactly as your doctor has told you. If you are not sure, ask your doctor, pharmacist, or nurse. The doctor will start by recommending a diet and exercise program. Follow these recommendations during treatment with Plyzari.
How much medicine to inject
Adults
Treatment starts with a low dose, which is gradually increased over the first five weeks of treatment.
- The initial dose of Plyzari is 0.6 mg once a day for at least one week.
- The dose should be increased gradually by 0.6 mg, according to the doctor's instructions, usually every week, until the recommended dose of 3.0 mg once a day is reached. The doctor will provide information on how much Plyzari to take in the following weeks of therapy. The recommended dosing schedule is usually as shown in the table below.
Week
Injected dose
Week 1
0.6 mg once a day
Week 2
1.2 mg once a day
Week 3
1.8 mg once a day
Week 4
2.4 mg once a day
From week 5
3.0 mg once a day
After reaching the recommended dose of 3.0 mg in week 5, it should be maintained until the end of therapy. Do not increase the dose further.
The doctor will regularly assess the treatment results.
Adolescents (≥ 12 years)
In the case of adolescents aged 12 to 18, a similar dose escalation schedule should be used as for adults (see the table above for adults). The dose should be increased gradually until a dose of 3.0 mg (maintenance dose) or the maximum tolerated dose is reached. Daily doses greater than 3.0 mg are not recommended.
How and when to use Plyzari
- Before the first use of the pen, the doctor or nurse will show you how to use it.
- Plyzari can be taken at any time of day, with or without food and drink.
- Take Plyzari at approximately the same time every day - you can choose the most convenient time for you.
Injection site
Plyzari should be injected under the skin (subcutaneous injection).
- The best injection sites are the front of the abdomen (stomach), the front of the thigh, or the upper arm.
- Do not inject into a vein or muscle.
The pen does not contain needles. It can be used with, for example, single-use BD Ultra-Fine or NovoFine needles with a thickness of 32 G and a length of up to 8 mm.
Detailed instructions are on the back of this leaflet.
Patients with diabetes
If you have diabetes, tell your doctor. To prevent low blood sugar, your doctor may need to adjust the dose of your anti-diabetic medicines.
- Do not mix Plyzari with other injectable medicines (e.g., insulin).
- Do not use Plyzari in combination with other medicines that are GLP-1 receptor agonists (such as exenatide or lixisenatide).
Using a higher dose of Plyzari than recommended
If you use a higher dose of Plyzari than recommended, contact your doctor or go to the hospital immediately. Take the packaging with you. You may need treatment. The following symptoms may occur:
- Nausea (vomiting)
- Vomiting
- Low blood sugar (hypoglycemia). Symptoms of low blood sugar - see "Possible side effects" in section 4.
Missing a dose of Plyzari
- If it has been less than 12 hours since the scheduled time for using Plyzari, use it as soon as possible.
- If it has been more than 12 hours since the time when you should have used Plyzari, skip the missed dose and take the next dose as usual the next day.
- Do not take a double dose or increase the dose the next day to make up for the missed dose.
Stopping treatment with Plyzari
Do not stop using this medicine without consulting your doctor.
If you have any further questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Severe side effects
Severe allergic reactions (anaphylaxis) have been rarely reported in patients treated with Plyzari.
Inflammation of the pancreas (pancreatitis) has been uncommonly reported in patients treated with Plyzari. Pancreatitis can be severe and potentially life-threatening.
Stop using this medicine and contact your doctor immediately if you experience any of the following severe side effects:
- Severe and persistent abdominal pain (in the stomach area) that may radiate to the back, as well as nausea and vomiting, as these may be symptoms of pancreatitis.
Other side effects
Very common:may affect more than 1 in 10 people
- Nausea (vomiting), vomiting, diarrhea, constipation, headache - these symptoms usually go away after a few days or weeks.
Common:may affect up to 1 in 10 people
- Gastrointestinal disorders, such as indigestion (dyspepsia), gastritis, stomach discomfort, upper abdominal pain, heartburn, bloating, gas, belching, and dry mouth
- Feeling weak or tired
- Taste disorders
- Dizziness
- Difficulty sleeping (insomnia), usually occurring during the first 3 months of treatment
- Gallstones
- Rash
- Reactions at the injection site (such as bruising, pain, redness, itching, and rash)
- Low blood sugar (hypoglycemia). Symptoms of low blood sugar may appear suddenly. They include: cold sweats, pale skin, headache, rapid heartbeat, nausea, feeling of extreme hunger, vision problems, drowsiness, feeling weak, nervousness, anxiety, confusion, difficulty concentrating, and shakiness. Your doctor will provide information on how to treat symptoms of low blood sugar and what to do if they occur.
- Increased activity of pancreatic enzymes, such as lipase and amylase.
Uncommon:may affect up to 1 in 100 people
- Fluid loss (dehydration). The occurrence of these symptoms is more likely at the beginning of treatment and may be caused by vomiting, nausea, and diarrhea
- Delayed gastric emptying
- Gallbladder inflammation
- Allergic reactions, including skin rash
- General feeling of being unwell
- Rapid heartbeat.
Rare:may affect up to 1 in 1,000 people
- Kidney dysfunction
- Acute kidney failure, symptoms may include decreased urine output, metallic taste in the mouth, and easy bruising.
Frequency not known:frequency cannot be estimated from the available data
- Intestinal obstruction. A severe form of constipation with additional symptoms such as abdominal pain, bloating, vomiting, etc.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Adverse Reaction Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Plyzari
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label of the pen and the outer packaging after EXP. The expiry date refers to the last day of the month stated.
Before first use:
Store in a refrigerator (2°C – 8°C). Do not freeze.
After opening the pen:
The pen can be stored for up to 1 month at a temperature below 30°C or in a refrigerator (2°C – 8°C). Do not freeze.
If the pen is not in use, to protect it from light, put the pen cap back on.
Do not use this medicine if you notice that the solution is not clear and colorless or almost colorless.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Plyzari contains
- The active substance is liraglutide. 1 mL of the solution for injection contains 6 mg of liraglutide. One pre-filled pen contains 18 mg of liraglutide.
- The other ingredients are: disodium citrate dihydrate, propylene glycol, phenol, and water for injections. Additionally, hydrochloric acid and/or sodium hydroxide may be added to adjust the pH.
What Plyzari looks like and contents of the pack
Plyzari is a clear and colorless or almost colorless solution for injection in a pre-filled pen. Each pen contains 3 mL of solution and allows for the administration of doses of 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg, and 3.0 mg.
Plyzari is available in packs containing 1, 3, or 5 pens. Not all pack sizes may be marketed.
The pack does not contain needles.
Marketing authorization holder and importer
Marketing authorization holder
Zentiva k.s.
U kabelovny 130
Dolní Měcholupy
102 37 Prague 10
Czech Republic
Importer
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park
Paola PLA 3000
Malta
This medicine is authorized in the Member States of the European Economic Area under the following names
Austria: Luntin 6 mg/ml Injektionslösung in einem Fertigpen
Spain: Plyzari 6mg/ml solución inyectable en pluma precargada
France: LIENDAX 6 mg/mL, solution injectable en stylo prérempli
Netherlands: Nevolat 6 mg/ml oplossing voor injectie in een voorgevulde pen
Hungary: Nevolat 6 mg/ml oldatos injekció előretöltött injekciós tollban
Germany, Norway, Sweden: Nevolat
Czech Republic, Poland, Portugal, Italy: Plyzari
For more information about this medicine, contact the local representative of the marketing authorization holder:
Zentiva Polska Sp. z.o.o.
ul. Bonifraterska 17
00-203 Warsaw, Poland
tel.: +48 22 375 92 00
Date of last revision of the leaflet:
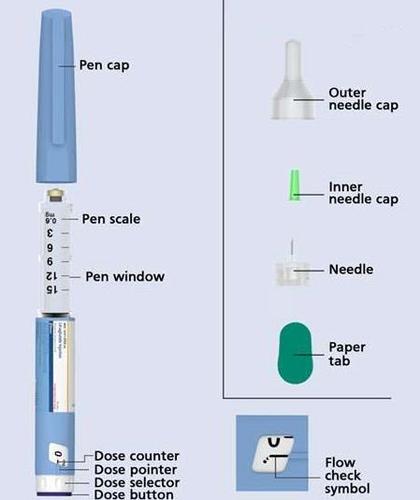
INSTRUCTIONS FOR USE OF THE PLYZARI PEN
Instructions for using Plyzari,
6 mg/mL, solution for injection in a pre-filled pen
Before using the Plyzari pen
read the following instructions carefully.
Do not use the pen without
proper trainingfrom a doctor or nurse.
Check the name and color of the labelon the pen to make sure it contains Plyzari. This is especially important if you are using several types of injectable medicines. Using the wrong medicine can be hazardous to your health.
Check that the solution in the pen is
clearand colorless. Look through the pen window. If the solution is cloudy, do not use the pen.
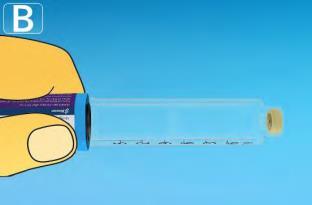
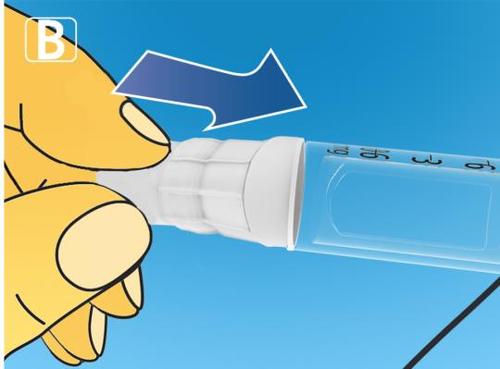
| Take a new needle and remove the paper tab. |  |
| Put the needle on the pen directly. Screw it on until it is securely attached. |  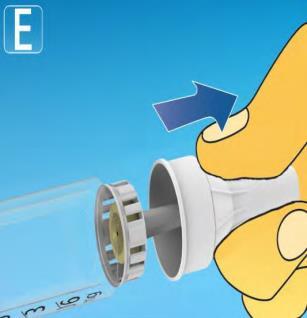 |
|
| How much solution is left? |  |
| 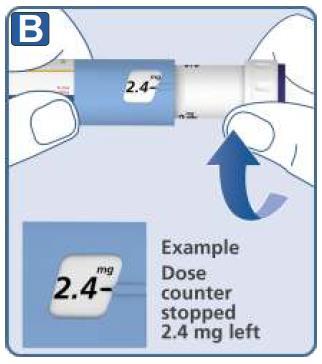 |
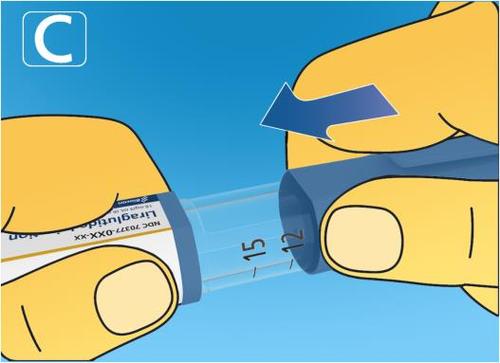
4. Injecting the dose
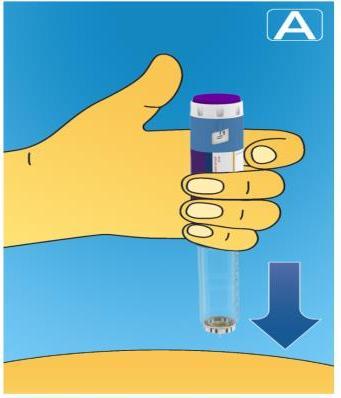
- Insert the needle under the skinas instructed by your doctor or nurse.
- Make sure the dose counter is visible.Do not cover it with your fingers. This may interrupt the injection.
Calculations should be done very carefully.
If you are unsure how to divide the dose between two pens, choose and inject the needed dose using a new pen.

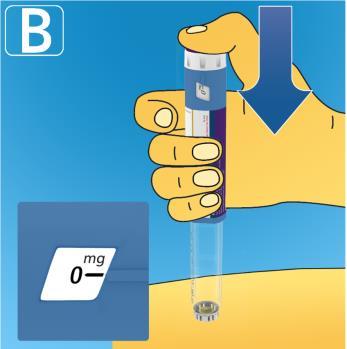
- Press and hold the dose button.
Watch the dose counter return to 0.
The 0 must be in line with the dose indicator. You may hear or feel a click.
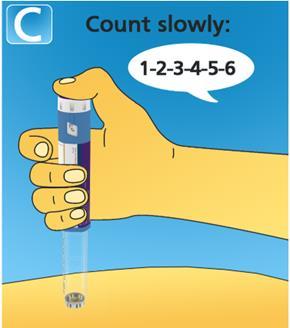
- Hold the needle in the skin for6 seconds after the dose counter has returned to 0.
- If you remove the needle before 6 seconds have passed, you may see solution leaking from the needle tip. This means you have not received a full dose.
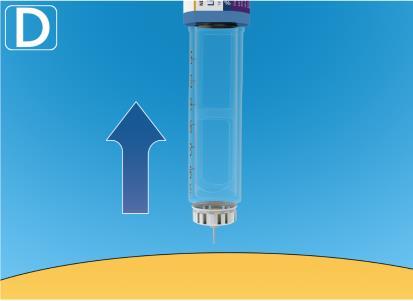
- Remove the needle from the skin.If blood appears at the injection site, apply gentle pressure. Do not rub the injection site.
After injection, a drop of solution may appear at the needle tip. This is normal and does not affect the dose administered.
Always check the dose counter to control the amount of medicine injected.
Hold the dose button down until the dose counter shows 0.
How to check if the needle is not blocked or damaged?
- If the dose counter does not show 0 after holding the dose button down, the needle may be blocked or damaged.
- This means that no medicinehas been injected at all, even if the dose counter has moved and shows a different dose than the one initially set.
What to do in case of a blocked needle?
Change the needle as described in step 5 "After injection" and repeat all steps starting from step 1 "Preparing the pen with a new needle". Make sure to select the full needed dose.
Do not touch the dose counter during injection.
This may interrupt the injection.
5. After injection

- Insert the needle into the outer needle capon a flat surface, without touching the needle or the outer needle cap.
|  |
 |  |
|
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterPharmadox Healthcare Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PlizariDosage form: Solution, 6 mg/mlActive substance: liraglutidePrescription requiredDosage form: Solution, 6 mg/mlActive substance: liraglutidePrescription requiredDosage form: Tablets, 500 mgActive substance: metforminPrescription required
Alternatives to Plizari in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Plizari in Spain
Alternative to Plizari in Ukraine
Online doctors for Plizari
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Plizari – subject to medical assessment and local rules.











