
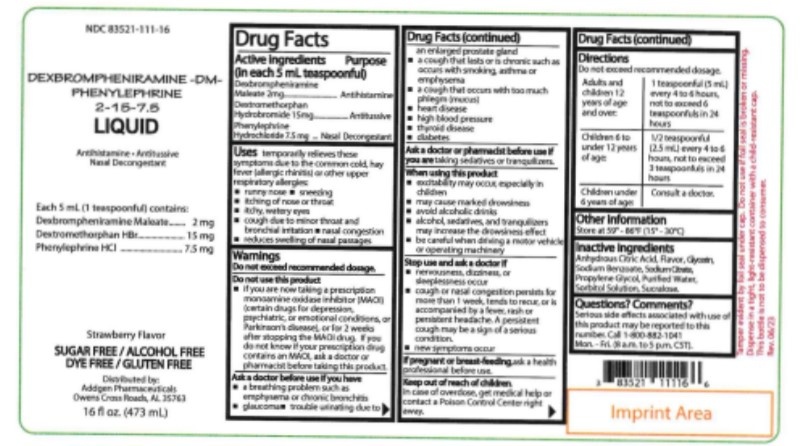
Phenilephrine Unimedic

Ask a doctor about a prescription for Phenilephrine Unimedic

How to use Phenilephrine Unimedic
Leaflet accompanying the packaging: information for the user
Phenylephrine Unimedic, 10 mg/ml,
concentrate for solution for injection/infusion
Phenylephrinum
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Phenylephrine Unimedic and what is it used for
- 2. Important information before using Phenylephrine Unimedic
- 3. How to use Phenylephrine Unimedic
- 4. Possible side effects
- 5. How to store Phenylephrine Unimedic
- 6. Contents of the packaging and other information
1. What is Phenylephrine Unimedic and what is it used for
This medicine belongs to the group of adrenergic and dopaminergic drugs. Phenylephrine Unimedic is used to treat low blood pressure, which may occur during anesthesia of various types.
2. Important information before using Phenylephrine Unimedic
When not to use Phenylephrine Unimedic:
- if the patient is allergic (hypersensitive) to phenylephrine or any of the other ingredients of this medicine (listed in section 6);
- if the patient has arterial hypertension (high blood pressure);
- if the patient has peripheral vascular disease (poor blood circulation);
- if the patient is taking non-selective monoamine oxidase inhibitors (MAOIs) used to treat depression (iproniazid, nialamide), or within 2 weeks of their discontinuation;
- if the patient has severe hyperthyroidism (thyrotoxicosis).
Warnings and precautions
Before starting treatment with Phenylephrine Unimedic, you should discuss it with your doctor, pharmacist, or nurse:
- if the patient is elderly;
- if the patient has hyperthyroidism;
- if the patient has heart disorders, such as weak pulse, heart block (partial), heart muscle disease, poor blood circulation in the heart, mild peripheral vascular disease, arrhythmias, tachycardia (fast heart rate), bradycardia (slow heart rate), angina pectoris;
- if the patient has poor blood circulation in the brain;
- if the patient has atherosclerosis (hardening and thickening of blood vessel walls);
- if the patient has diabetes;
- if the patient is using oxytocin - the effect on blood vessels may be enhanced and cause very high blood pressure and stroke in the postpartum period;
- if the patient has arterial hypertension;
- if the patient has closed-angle glaucoma.
In patients with severe heart failure, phenylephrine may increase heart failure due to vasoconstriction. During treatment, blood pressure will be monitored. In patients with heart disease, additional monitoring of vital functions will be performed.
Children
The medicine is not recommended for use in children due to the lack of sufficient data on efficacy, safety, and dosage.
Phenylephrine Unimedic and other medicines
You should tell your doctor, pharmacist, or nurse about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take. You should not use Phenylephrine Unimedic:
- with iproniazid, nialamide (antidepressants).
The following medicines may affect the action of Phenylephrine Unimedic or Phenylephrine Unimedic may affect their action when used concomitantly:
- dihydroergotamine, ergotamine, methylergometrine, methysergide (medicines used for migraine);
- linezolid (antibiotic);
- bromocriptine, cabergoline, lisuride, pergolide (medicines used for Parkinson's disease);
- desipramine, imipramine, nortriptyline, moclobemide, toloxatone, minalcipran, venlafaxine (antidepressants);
- inhalational anesthetics (desflurane, enflurane, halothane, isoflurane, methoxyflurane, sevoflurane);
- medicines used to treat high blood pressure (guanethidine);
- medicines used to treat heart failure and irregular heartbeat (cardiac glycosides);
- medicines used to treat arrhythmias (quinidine);
- medicines used during childbirth (oxytocin).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor, pharmacist, or nurse before using this medicine. Pregnancy This medicine should not be used during pregnancy, unless it is absolutely necessary. Breastfeeding This medicine should not be used during breastfeeding, unless it is absolutely necessary. However, in the case of a single administration during childbirth, breastfeeding is possible.
Driving and using machines
This is not applicable.
Phenylephrine Unimedic contains sodium
Each 2 ml ampoule (containing 1 ml of concentrate) contains 0.2 mmol (3.7 mg) of sodium. The medicine contains less than 1 mmol (23 mg) of sodium, which means it is considered "sodium-free".
3. How to use Phenylephrine Unimedic
The medicine is administered only by healthcare professionals with appropriate training and experience. Administration in adults The doctor or nurse administers Phenylephrine Unimedic intravenously. The doctor will determine the appropriate dose for the patient, the duration of administration, and the method of administration. Administration in patients with renal impairment In patients with renal impairment, smaller doses of phenylephrine may be necessary. Administration in patients with hepatic impairment In patients with liver cirrhosis, larger doses of phenylephrine may be necessary. Administration in the elderly Treatment of the elderly should be carried out with caution. Administration in children The use of Phenylephrine Unimedic is not recommended in children due to insufficient data on safety, efficacy, and recommended dose.
Overdose of Phenylephrine Unimedic
Symptoms of overdose of Phenylephrine Unimedic include: faster and irregular heartbeat, headaches, nausea, vomiting, paranoid psychosis, hallucinations, and arterial hypertension (headache, shortness of breath, fatigue). However, it is unlikely to occur, as the medicine is administered in a hospital setting.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects have been reported with an unknown frequency. Some side effects may be serious.
You should immediately inform your doctor if you experience any of the following side effects:
side effects:
- chest pain or pain associated with angina,
- irregular heartbeat,
- palpitations in the chest,
- brain hemorrhage (speech disorders, dizziness, weakness on one side of the body),
- psychosis (loss of contact with reality).
Other side effects may include (frequency unknown):
- hypersensitivity reactions (allergy),
- excessive pupil dilation,
- increased intraocular pressure (exacerbation of glaucoma),
- irritability (increased sensitivity of an organ or body part),
- excitement (anxiety),
- anxiety,
- disorientation,
- headache,
- nervousness,
- insomnia (difficulty falling asleep and sleeping),
- tremors,
- skin burning,
- skin tingling,
- feeling of itching or tingling of the skin (paresthesia),
- slow or fast heart rate,
- high blood pressure (headache, shortness of breath, fatigue)
- breathing difficulties,
- fluid in the lungs,
- nausea,
- vomiting,
- sweating,
- pallor (pale skin color),
- goosebumps,
- tissue damage at the injection site,
- muscle weakness,
- difficulty urinating or urinary retention.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products Al. Jerozolimskie 181 C 02-222 Warsaw Tel.: +48 22 49 21 301 Fax: + 48 22 49 21 309 website: https://smz.ezdrowie.gov.pl Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Phenylephrine Unimedic
Store the ampoules in the outer packaging to protect them from light. Storage conditions after dilutionChemical and physical stability has been demonstrated during use for 7 days at room temperature (20 to 25°C) From a microbiological point of view, the product should be used immediately. Otherwise, the user is responsible for the storage time and conditions of the prepared solution. The storage time should not normally exceed 24 hours at a temperature of 2°C to 8°C, unless the dilution was performed under controlled and validated aseptic conditions. The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the carton after EXP. The expiry date refers to the last day of the month stated. Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Phenylephrine Unimedic contains
- The active substance is phenylephrine. Phenylephrine Unimedic contains 10 mg/ml of phenylephrine (as phenylephrine hydrochloride).
- The other ingredients are sodium chloride, sodium citrate, anhydrous citric acid, water for injections, and hydrochloric acid (to adjust pH) and sodium hydroxide (to adjust pH).
What Phenylephrine Unimedic looks like and contents of the pack
Clear, colorless solution. Phenylephrine Unimedic, 10 mg/ml, concentrate for solution for injection/infusion is available in glass ampoules (2 ml) containing 1 ml of solution. Packs of 5, 10, 20, 50, or 100 ampoules in a cardboard box. Not all pack sizes may be marketed.
Marketing authorization holder
Unimedic Pharma AB Box 6216 102 34 Stockholm Sweden
Manufacturer
Unimedic AB Storjordenvägen 2 SE-864 31 Matfors Sweden
Date of last revision of the leaflet:
The following information is intended for healthcare professionals only: The solution has a high concentration and must be diluted before administration.Reconstitution/dilution: Phenylephrine Unimedic 10 mg/ml is administered as an intravenous injection or infusion, after dilution with sodium chloride 9 mg/ml solution (or glucose 50 mg/ml solution).
- Dilution to a concentration of 100 micrograms/ml: 1 ml of the 10 mg/ml solution is diluted with 100 ml of sodium chloride 9 mg/ml solution (or glucose 50 mg/ml solution).
- Dilution to a concentration of 50 micrograms/ml: 1 ml of the 10 mg/ml solution is diluted with 200 ml of sodium chloride 9 mg/ml solution (or glucose 50 mg/ml solution). Other concentrations may be used.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterUnimedic AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Phenilephrine UnimedicDosage form: Solution, 0.1 mg/mlActive substance: phenylephrineManufacturer: Sintetica GmbH Sirton Pharmaceuticals SpA.Prescription not requiredDosage form: Solution, 10 mg/mlActive substance: phenylephrineManufacturer: Sintetica GmbH Sirton Pharmaceuticals SpA.Prescription not requiredDosage form: Solution, 100 micrograms/mlActive substance: phenylephrineManufacturer: Laboratoire AguettantPrescription not required
Alternatives to Phenilephrine Unimedic in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Phenilephrine Unimedic in Ukraine
Alternative to Phenilephrine Unimedic in Spain
Online doctors for Phenilephrine Unimedic
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Phenilephrine Unimedic – subject to medical assessment and local rules.










