
Phenazolinum

How to use Phenazolinum
Leaflet attached to the packaging: patient information
PHENAZOLINUM, 50 mg/ml, solution for injections
Antazolinemesylate
Please read the contents of the leaflet carefully before using the medicine, as it contains important information for the patient.
- Please keep this leaflet, so you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed to a specific person. Do not pass it on to others. The medicine may harm another person, even if their illness symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Phenazolinum and what is it used for
- 2. Important information before using Phenazolinum
- 3. How to use Phenazolinum
- 4. Possible side effects
- 5. How to store Phenazolinum
- 6. Package contents and other information
1. What is Phenazolinum and what is it used for
Phenazolinum contains antazoline, which is an antihistamine. It inhibits allergic symptoms, especially those related to histamine release, and also has antiarrhythmic properties.
The medicine is used:
- as an adjunct in acute allergic conditions, including anaphylactic shock in combination with adrenaline,
- in paroxysmal arrhythmias of supraventricular origin, including atrial fibrillation, unresponsive to standard antiarrhythmic treatment.
2. Important information before using Phenazolinum
When not to use Phenazolinum
Warnings and precautions
Before starting to use Phenazolinum, you should discuss it with your doctor or pharmacist.
Antazoline should be avoided in patients with epilepsy due to reports of seizures during treatment with antihistamines.
In patients with hypertension, fixed arrhythmias, diabetes, hyperthyroidism, and prostatic hyperplasia, antazoline should be used with caution.
Antazoline should be avoided if the patient is taking monoamine oxidase inhibitors (MAOIs), cholinolytic drugs (phenothiazine neuroleptics, hydroxyzine, tricyclic antidepressants).
Concomitant use of antazoline and central nervous system depressants, as well as alcohol consumption, may lead to additive effects and cause dangerous symptoms.
Children
The medicine should not be used in children under 12 years of age.
Phenazolinum and other medicines
You should tell your doctor or pharmacist about all the medicines you are taking or have recently taken, as well as any medicines you plan to take.
The action and toxicity of antazoline are enhanced by concomitantly used barbiturates, monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, noradrenaline, adrenaline, and alcohol.
Antazoline weakens the action of phenytoin, oral anticoagulants, and steroids.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
The medicine may be used during pregnancy only if, in the doctor's opinion, the benefit to the mother outweighs the potential risk to the fetus.
You should not breastfeed while using antazoline.
Driving and operating machinery
The medicine may impair psychophysical abilities - during its use, you should not drive vehicles or operate machinery.
Phenazolinum contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per 2 ml of solution, which means the medicine is considered "sodium-free".
3. How to use Phenazolinum
This medicine should always be used as directed by your doctor or pharmacist. If you have any doubts, you should consult your doctor or pharmacist.
Each ampoule contains 100 mg of antazoline mesylate.
Allergic reactions
Adults
Intramuscularly from 200 mg to 300 mg (2 to 3 ampoules) per day.
Adolescents over 12 years of age
Intramuscularly from 50 mg to 100 mg (half to 1 ampoule).
Atrial fibrillation
Adults
Administer intravenously under strict control of blood pressure and heart function (ECG), in a dose of 100 mg to 300 mg (1 to 3 ampoules) over 3-10 minutes. Injections should be stopped when sinus rhythm returns.
Larger doses should be used in the intensive care unit.
Instructions for opening the ampoule
Before opening the ampoule, make sure the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
A colored dot is placed on each ampoule (see figure 1) as a mark indicating the break point below it.
- To open the ampoule, hold it vertically, with both hands, with the colored dot facing you - see figure 2. The upper part of the ampoule should be grasped so that the thumb is above the colored dot.
- Press according to the arrow on figure 3. The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused product should be destroyed in accordance with applicable regulations. Figure 1 Figure 2 Figure 3
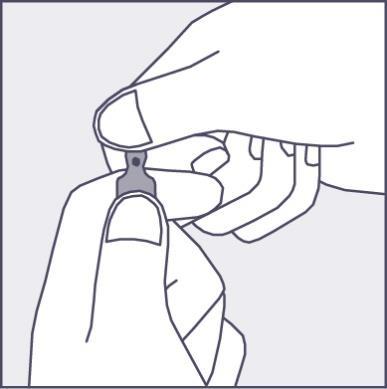
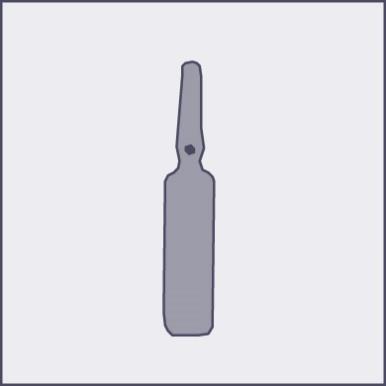
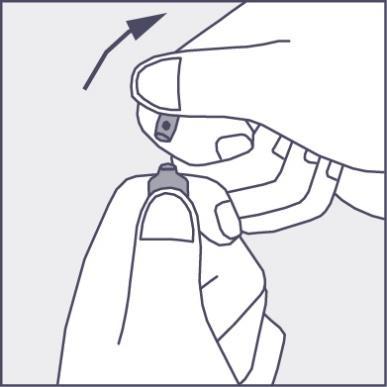
Using a larger dose of Phenazolinum than recommended
There may be increased difficulty breathing leading to respiratory arrest, impaired consciousness, coma, decreased body temperature (in children), tremors, and seizures. Symptomatic treatment.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Antazoline may cause gastrointestinal disorders (nausea, vomiting, diarrhea), increased blood pressure, arrhythmias, weakness, drowsiness, fatigue, concentration disorders, anxiety.
Rarely, it may cause hemolytic anemia, hemoglobinuria (red urine discoloration), and acute renal failure as a result of immunological disorders after antazoline.
It may cause confusion, coordination disorders, dry mouth, vision disturbances, difficulty urinating.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Phenazolinum
Store at a temperature below 25°C. Do not freeze.
Store in the original packaging to protect from light.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste containers. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Phenazolinum contains
- The active substance of the medicine is antazoline mesylate. Each ml of solution contains 50 mg of antazoline mesylate.
- The other ingredients are: disodium edetate, calcium sodium edetate, water for injections.
One ampoule (2 ml of solution) contains 100 mg of antazoline mesylate.
What Phenazolinum looks like and what the package contains
Ampoules made of colorless glass in a PVC blister pack in a cardboard box.
10 ampoules of 2 ml each
Marketing authorization holder and manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of last update of the leaflet:December 2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
Talk to a doctor online
Need help understanding this medicine or your symptoms? Online doctors can answer your questions and offer guidance.














