
Pedicetamol


How to use Pedicetamol
Leaflet attached to the packaging: information for the user
Pedicetamol, 100 mg/ml, oral solution
Paracetamol
Read the leaflet carefully before taking the medicine, as it contains
important information for the patient.
This medicine should always be taken exactly as described in the patient leaflet or as advised by the doctor, pharmacist, or nurse.
- The leaflet should be kept in case it needs to be read again.
- In case of any doubts, the pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform the doctor, pharmacist, or nurse. See section 4.
- If there is no improvement or the patient feels worse, if the fever does not subside after 3 days, or if the pain does not subside after 3 days in children or 5 days in adults (2 days in the case of throat pain), the doctor should be contacted.
Table of contents of the leaflet
- 1. What is Pedicetamol and what is it used for
- 2. Important information before taking Pedicetamol
- 3. How to take Pedicetamol
- 4. Possible side effects
- 5. How to store Pedicetamol
- 6. Contents of the packaging and other information
1. What is Pedicetamol and what is it used for
Pedicetamol belongs to a group of pain-relieving and antipyretic medicines. The medicine is used to reduce fever lasting no longer than 3 days and to relieve pain of mild to moderate severity.
If there is no improvement or the patient feels worse, the fever does not subside after 3 days, or the pain does not subside after 3 days in children or 5 days in adults (2 days in the case of throat pain), the doctor should be consulted.
2. Important information before taking Pedicetamol
When not to take Pedicetamol
- if the patient is allergic to paracetamol or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
- before starting to take Pedicetamol, it should be discussed with the doctor, pharmacist, or nurse;
- the doctor should be consulted before giving this medicine to children under 3 years of age;
- a dose larger than the recommended dose should not be given;
- if the patient has kidney, heart, or lung disorders, or if the patient has anemia (reduced hemoglobin levels in the blood, related or not to a reduced number of red blood cells), or if the activity of the enzyme glucose-6-phosphate dehydrogenase in the blood is low, the doctor should be consulted before taking the medicine;
in the case of liver disease (including Gilbert's syndrome), the doctor should be consulted to reduce the dose and (or) increase the intervals between consecutive doses;
- in patients with a body weight below 50 kg due to anorexia, malnutrition, or dehydration, due to the possibility of increased hepatotoxicity;
- drinking alcoholic beverages while taking paracetamol may cause liver damage;
- if there is a high fever (>39°C), the doctor should be consulted before taking Pedicetamol;
- if the pain persists for more than 3 days in children or 5 days in adults (2 days in the case of throat pain) or if the fever persists for more than 3 days, symptoms worsen, or other symptoms appear, treatment should be discontinued and the doctor consulted;
- while taking Pedicetamol, the doctor should be informed immediately if the patient has severe illnesses, including severe kidney disorders or sepsis (when bacteria and their toxins circulate in the blood, leading to organ damage) or malnutrition, chronic alcoholism, or if the patient is also taking flucloxacillin (an antibiotic). Severe metabolic acidosis (a blood and fluid disorder) has been reported in patients who take paracetamol in regular doses for a longer period or when taking paracetamol with flucloxacillin. Symptoms of metabolic acidosis may include: severe breathing difficulties, including rapid deep breathing, drowsiness, feeling of nausea (nausea) and vomiting.
Pedicetamol with other medicines
The doctor or pharmacist should be informed about all medicines the patient is currently taking or has recently taken, as well as any medicines the patient plans to take.
In particular, in the case of medicines containing any of the following active substances, it may be necessary to change the dose or stop taking one of the following medicines:
- antibiotics (chloramphenicol);
- oral anticoagulants (acenocoumarol, warfarin);
- oral contraceptives and estrogens;
- antiepileptic drugs (lamotrigine, phenytoin, or other hydantoins, phenobarbital, methylphenobarbital, primidone, carbamazepine);
- antituberculosis drugs (isoniazid, rifampicin);
- barbiturates (used as sedatives, tranquilizers, and anticonvulsants);
- activated charcoal, used to treat diarrhea and bloating;
- cholestyramine (used to reduce blood cholesterol levels);
- drugs used to treat gout (probenecid and sulfinpyrazone);
- drugs used to relieve spasms and cramps of the stomach, intestine, and bladder (anticholinergic drugs);
- metoclopramide and domperidone (used to prevent nausea and vomiting);
- propranolol used to treat high blood pressure (hypertension) and heart rhythm disorders (arrhythmia);
- zidovudine (used to treat people infected with the human immunodeficiency virus that causes AIDS);
- flucloxacillin (an antibiotic) due to a serious blood and fluid disorder (called metabolic acidosis), which requires emergency treatment (see section 2).
Effect on laboratory tests:
In the case of planned laboratory tests (such as blood tests, urine tests, skin allergy tests, etc.), the doctor should be informed about the use of this medicine, as it may affect the results of these tests.
Pedicetamol with food, drink, and alcohol
Pedicetamol can be diluted with water, milk, or fruit juices. Taking paracetamol in patients who regularly drink alcohol (three or more alcoholic beverages per day) may damage the liver.
Pregnancy, breastfeeding, and fertility
Before taking any medicine, the doctor or pharmacist should be consulted.
Pedicetamol can be given to pregnant women if necessary. The smallest effective dose should be used to relieve pain or reduce fever, and the medicine should be taken for as short a time as possible. If the pain is not relieved or the fever does not subside, or if it is necessary to increase the frequency of taking the medicine, the doctor should be consulted.
Paracetamol can be used in therapeutic doses during pregnancy and breastfeeding.
Driving and using machines
No symptoms have been described that could affect the ability to drive vehicles or operate machines.
Pedicetamol contains azorubine and sodium (from sodium saccharin)
The medicine may cause allergic reactions because it contains azorubine (E122).
The medicine contains less than 1 mmol (23 mg) of sodium per 1 ml, which means the medicine is considered "sodium-free".
3. How to take Pedicetamol
This medicine should always be taken exactly as described in the patient leaflet or as advised by the doctor, pharmacist, or nurse. In case of doubts, the doctor, pharmacist, or nurse should be consulted.
Pedicetamol is intended for use in children with a body weight of up to 32 kg (approximately from 0 months to 10 years). It is necessary to follow the dosing based on the child's body weight and determine the appropriate dose of the oral solution in milliliters.
Approximate age ranges given in relation to body weight are provided only as additional information for the patient.
The recommended daily dose of paracetamol is approximately 60 mg/kg body weight per day and is given in 4 to 6 doses per day, e.g., 15 mg/kg body weight every 6 hours or 10 mg/kg body weight every 4 hours.
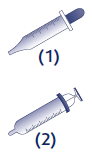
In children under 3 yearsof age, it is recommended to give the oral solution in the form of drops (4 mg/drop) using a dropper (1) attached to the 30 ml packaging.
In children from 3 yearsof age, it is recommended to give the oral solution in milliliters (ml) using a syringe (2) attached to the 60 ml packaging.
Below is the instruction for administering the medicine in a dose of 15 mg/kg every 6 hours:
Child's body weight
Age (approximate)
Volume in ml
mg
of paracetamol
Equivalent in drops
up to 4 kg
from 0 to 3 months
0.6 ml
60 mg
15 drops up to 7 kg
from 4 to 8 months
1.0 ml
100 mg
25 drops up to 8 kg
from 9 to 11 months
1.2 ml
120 mg
30 drops up to 10.5 kg
from 12 to 23 months
1.6 ml
160 mg
40 drops up to 13 kg
from 2 to 3 years
2.0 ml
200 mg
- - up to 18.5 kgfrom 4 to 5 years
2.8 ml
280 mg
- - up to 24 kgfrom 6 to 8 years
3.6 ml
360 mg
- -
up to 32 kg
from 9 to 10 years
4.8 ml
480 mg
To determine the dose directly, the child's body weight in kilograms should be multiplied by 0.15.
The result is the number of ml of Pedicetamol to be given.
In children, this dose should be given every 6 hours, also at night.
If the desired effect is not achieved within 3-4 hours, the medicine may be given every 4 hours. In this case, a dose of 10 mg/kg body weight should be given.
In infants with a body weight below 7 kg (6 months), it is recommended to use suppositories (if available), except in cases where administration of this pharmaceutical form is impossible for clinical reasons (e.g., diarrhea).
A dose larger than the maximum daily dose of 60 mg/kg/day should never be given without prior consultation with the doctor.
Patients with liver or kidney disease, see section 2, Warnings and precautions.
Administration of the medicine depends on the occurrence of pain and fever symptoms. If the symptoms subside, administration of the medicine should be discontinued.
Instruction for proper administration of the product
Pedicetamol is administered orally.
The 30 ml bottle with a child-resistant 2 ml dropper:
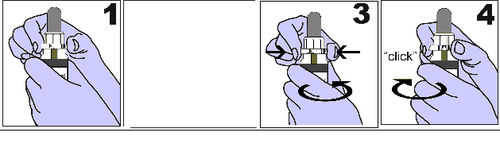
1 and 2. - The bottle should be held firmly in one hand. With the other hand, the cap should be grasped with the thumb and index finger in the place marked with triangles on the cap, where the inscription "PRESS" is located.
- 3. - To open the bottle, press the triangle and unscrew the cap by turning it to the left (counterclockwise). The required amount of solution should be drawn using the dropper. It can be administered directly or diluted with water.
- 4. - To close the bottle, the cap should be screwed to the right (clockwise) until a click is heard. The bottle should be closed tightly after each use.
The 60 ml bottle with a child-resistant closure and a 5 ml oral syringe:
- 1. - Open the bottle according to the instructions on the cap (when opening for the first time, the one-time seal must be broken).
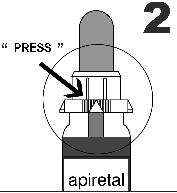
- 2. - Insert the oral syringe by pressing the perforated hole.
- 3. - Turn the bottle upside down and draw the appropriate amount of solution.
- 4. - Administer directly or diluted with water, milk, or juice.
- 5. - The oral syringe should be washed with water after use. The bottle should be closed tightly after each use.
Taking a larger dose of Pedicetamol than recommended
In the event of taking (administering to a child) a larger dose of paracetamol than recommended, the doctor or pharmacist should be consulted immediately and the name of the medicine and the amount taken should be provided.
It is helpful to take the packaging and leaflet with you and give them to the specialist.
In the event of an overdose, you should go to a health center as soon as possible, even if no symptoms have appeared, because even in severe poisoning, symptoms may not appear until up to 3 days later. Symptoms of an overdose may include: dizziness, vomiting, loss of appetite, yellowish skin and eye color (jaundice), and abdominal pain.
Paracetamol overdose is considered a situation in which an adult has taken a total of more than 6 g, and a child more than 100 mg per kilogram of body weight. Treatment of an overdose is more effective if started within 4 hours of taking the medicine.
Patients taking barbiturates and those with chronic alcoholism may be more susceptible to paracetamol overdose.
Generally, in the case of paracetamol overdose, treatment is symptomatic.
Missing a dose of Pedicetamol
A double dose should not be taken to make up for a missed dose.
If a dose is missed, the next dose should be taken as soon as possible, and then the medicine should be continued according to the normal schedule. However, if the time to take the next dose is very short, the missed dose should be skipped and the next dose taken according to the recommended dosing schedule.
Stopping Pedicetamol
In case of any further doubts about the use of the medicine, the doctor, pharmacist, or nurse should be consulted.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Adverse reactions to paracetamol are rare (occurring in 1 to 10 per 10,000 patients) or very rare (occurring in less than 1 per 10,000 patients).
Rare side effects include general malaise, hypotension, and increased liver enzyme activity.
Very rarely, when taking large doses of the medicine or during long-term treatment, liver damage may occur. Very rarely, hypoglycemia, cloudy urine, adverse effects on the kidneys, skin rash, hives, anaphylactic shock, and changes in blood cell count, such as neutropenia and leukopenia, may occur.
In very rare cases, serious skin reactions have been reported.
Frequency not known (cannot be estimated from the available data):
A serious condition that can make the blood more acidic (so-called metabolic acidosis), in patients with severe illness taking paracetamol (see section 2).
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C,
02-222 Warsaw,
Phone: +48 22 49 21 301,
Fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Pedicetamol
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiry date stated on the carton after: EXP. The expiry date refers to the last day of the month stated.
The bottle should be stored in the outer packaging. There are no special instructions for storing the medicine at a certain temperature.
After opening, the bottle should be stored in the outer packaging.
After opening, the contents of the bottle should be used within 6 months.
Medicines should not be disposed of via wastewater or household waste. The pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Pedicetamol contains
- The active substance of the medicine is paracetamol. Each ml of the solution contains 100 mg of paracetamol.
- The other ingredients (excipients) are: macrogol 600, glycerol, raspberry flavor, sodium saccharin (E-954), azorubine (carmoisine) (E122), and purified water.
What Pedicetamol looks like and what the packaging contains
Pedicetamol is a clear, red oral solution, packaged in plastic bottles of 30 and 60 ml capacity.
- - 30 ml bottle:a clear, plastic bottle with a child-resistant 2 ml dropper.
- - 60 ml bottle:a clear, plastic bottle with a child-resistant closure and a 5 ml oral syringe.
Marketing authorization holder and manufacturer
Marketing authorization holder:
"Polski Lek-Dystrybucja" Sp. z o.o.
Chopin Street 10,
34-100 Wadowice
Phone: +48 33 870 83 01
e-mail: pv@polskilek.pl
Manufacturer:
LABORATORIOS ERN, S.A.
Gorgs Lladó, 188
08210 Barberá del Vallés, Barcelona
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Spain
Apiredol 100 mg/ml oral solution
Germany
Apiredol 100 mg/ml solution for ingestion
France
Apedital 100 mg/ml oral solution
Apedital 100 mg/ml oral solution in drops
Italy
Apiredol 100 mg/ml oral solution
Poland
Pedicetamol
Portugal Paracetamol Ern 100 mg/ml oral solution
Date of last revision of the leaflet:April 2025.
Other sources of information
Detailed information about this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterLaboratorios ERN, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
Talk to a doctor online
Need help understanding this medicine or your symptoms? Online doctors can answer your questions and offer guidance.














