

Papaverinum hidrohloricum Vzf

Ask a doctor about a prescription for Papaverinum hidrohloricum Vzf

How to use Papaverinum hidrohloricum Vzf
Leaflet attached to the packaging: patient information
PAPAVERINUM HYDROCHLORICUM WZF, 20 mg/ml, solution for injections
Papaverine hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their illness symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Papaverinum hydrochloricum WZF and what is it used for
- 2. Important information before using Papaverinum hydrochloricum WZF
- 3. How to use Papaverinum hydrochloricum WZF
- 4. Possible side effects
- 5. How to store Papaverinum hydrochloricum WZF
- 6. Package contents and other information
1. What is Papaverinum hydrochloricum WZF and what is it used for
Papaverine belongs to the isoquinoline alkaloids of opium. It relaxes smooth muscles by directly affecting their cells. Papaverine does not have analgesic effects and does not lead to addiction.
The medicine is used in spastic conditions (muscle spasms) of:
- the digestive tract - biliary colic, spastic conditions of the bile ducts, intestinal colic;
- the urinary tract - renal colic, painful urination.
2. Important information before using Papaverinum hydrochloricum WZF
When not to use Papaverinum hydrochloricum WZF
- if the patient is allergic to papaverine or any other component of the medicine (listed in section 6);
- if the patient has conduction disorders in the heart muscle.
Warnings and precautions
Before starting to use Papaverinum hydrochloricum WZF, the patient should discuss it with their doctor, especially if they have:
- glaucoma,
- liver function disorders,
- angina pectoris,
- recently had a heart attack.
Children
Due to the lack of relevant clinical data, Papaverinum hydrochloricum WZF should not be used in children.
Papaverinum hydrochloricum WZF and other medicines
The patient should inform their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Papaverine weakens the effect of levodopa (a medicine used to treat Parkinson's disease).
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
The medicine contains benzyl alcohol - see below, section "Papaverinum hydrochloricum WZF contains benzyl alcohol and sodium."
The doctor will decide whether the medicine can be used.
Driving and operating machinery
The patient should be cautious, as the medicine may cause dizziness or drowsiness.
Papaverinum hydrochloricum WZF contains benzyl alcohol and sodium
The medicine contains 10 mg of benzyl alcohol in each ml of solution. Each ampoule (2 ml of solution) contains 20 mg of benzyl alcohol.
Benzyl alcohol may cause allergic reactions.
Administering benzyl alcohol to small children is associated with a risk of severe side effects, including breathing difficulties (so-called "gasping syndrome"). Papaverinum hydrochloricum WZF should not be used in children (see also section 3).
Pregnant or breastfeeding women, patients with liver or kidney diseases should consult their doctor before using the medicine, as a large amount of benzyl alcohol may accumulate in their body and cause side effects (so-called metabolic acidosis).
The medicine contains less than 1 mmol (23 mg) of sodium per ampoule (2 ml), which means the medicine is considered "sodium-free".
3. How to use Papaverinum hydrochloricum WZF
This medicine should always be used according to the doctor's recommendations. If you have any doubts, consult your doctor or pharmacist.
Adults
Administer intramuscularly or subcutaneously, without dilution:
from 40 mg to 120 mg (from 2 ml to 6 ml, i.e., 1 to 3 ampoules). If necessary, the dose may be repeated after 3 hours, up to 4 times a day.
Children
Papaverinum hydrochloricum WZF should not be used in children.
Instructions for opening the ampoule
Before opening the ampoule, make sure the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to help the solution flow down.
A colored dot is marked on each ampoule (see Figure 1) as a sign of the break point located below it.
- To open the ampoule, hold it vertically, with both hands, with the colored dot facing you - see Figure 2. Hold the upper part of the ampoule so that your thumb is above the colored dot.
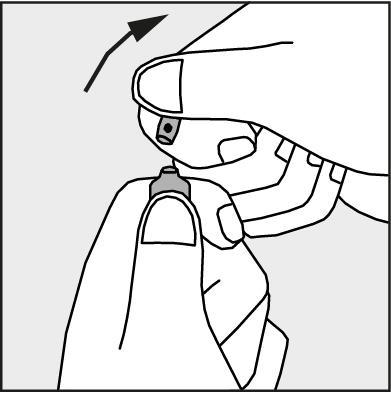
- Press according to the arrow shown in Figure 3.
The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused medicine should be disposed of in accordance with applicable regulations.
Figure 1.
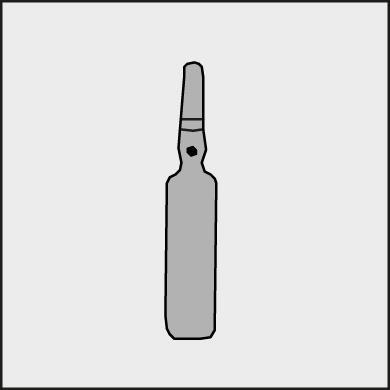
Figure 2.
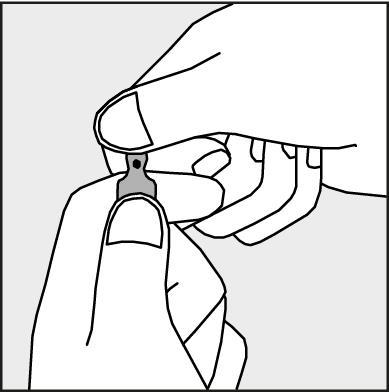
Figure 3.
Using a higher dose of Papaverinum hydrochloricum WZF than recommended
Excessive lowering of blood pressure, blurred or double vision, heart rhythm disorders may occur.
Treatment: the doctor will provide symptomatic treatment.
If a higher dose of the medicine is used than recommended, consult a doctor or pharmacist immediately.
Missing a dose of Papaverinum hydrochloricum WZF
If a dose of the medicine is missed, the next dose should be taken at the scheduled time. Do not take a double dose to make up for the missed dose.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Flushing of the face, pain and dizziness, discomfort in the abdominal cavity, constipation, diarrhea, malaise, loss of appetite, nausea, vomiting, excessive sweating, drowsiness, hypotension, breathing difficulties, pain at the injection site, eosinophilia, elevated liver test values.
If symptoms such as jaundice or liver pain occur, the medicine should be discontinued and a doctor consulted immediately.
Reporting side effects
If any side effects occur, including any side effects not listed in this leaflet, inform your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Papaverinum hydrochloricum WZF
Store at a temperature below 25°C. Store the ampoules in the outer packaging to protect them from light. Do not freeze.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of the specified month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste containers. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Papaverinum hydrochloricum WZF contains
- The active substance of the medicine is papaverine hydrochloride. Each ml of solution contains 20 mg of papaverine hydrochloride.
- The other ingredients are: disodium edetate, benzyl alcohol, water for injections.
What Papaverinum hydrochloricum WZF looks like and what the package contains
Papaverinum hydrochloricum WZF is a clear, colorless or slightly yellowish liquid.
10 ampoules of 2 ml each in a cardboard box.
Marketing authorization holder and manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of last update of the leaflet:December 2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Papaverinum hidrohloricum VzfDosage form: Suppositories, (15 mg + 40 mg)/1.5 gActive substance: papaverineManufacturer: Farmina Sp. z o.o.Prescription not requiredDosage form: Tablets, 80 mgActive substance: drotaverineManufacturer: mibe GmbH ArzneimittelPrescription not requiredDosage form: Tablets, 40 mgActive substance: drotaverineManufacturer: mibe GmbH ArzneimittelPrescription not required
Alternatives to Papaverinum hidrohloricum Vzf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Papaverinum hidrohloricum Vzf in Ukraine
Online doctors for Papaverinum hidrohloricum Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Papaverinum hidrohloricum Vzf – subject to medical assessment and local rules.














