
Palexia

How to use Palexia
Leaflet accompanying the packaging: patient information
Palexia, 20 mg/ml, oral solution
Tapentadol
Read the leaflet carefully before taking the medicine, as it contains important information for the patient
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Palexia and what is it used for
- 2. Important information before taking Palexia
- 3. How to take Palexia
- 4. Possible side effects
- 5. How to store Palexia
- 6. Contents of the packaging and other information
1. What is Palexia and what is it used for
Tapentadol - the active substance of Palexia - is a strong pain reliever belonging to the group of opioids. Palexia is indicated for the treatment of acute pain of moderate to severe intensity in children and adolescents over 2 years of age and with a body weight over 16 kg, as well as in adults, in whom only the use of an opioid analgesic allows for proper treatment.
2. Important information before taking Palexia
When not to take Palexia:
- in patients with asthma or dangerously slow and shallow breathing (respiratory depression, increased carbon dioxide levels in the blood),
- in patients with intestinal obstruction,
- in case of acute alcohol poisoning, sleeping pills, painkillers, or other psychotropic drugs (mood and emotion-affecting drugs) (see "Palexia and other medicines").
Warnings and precautions
Before starting treatment with Palexia, discuss it with your doctor, pharmacist, or nurse:
- in case of slow or shallow breathing,
- in case of increased intracranial pressure or consciousness disorders up to coma,
- in patients after head injury or with brain tumors,
- in patients with liver or kidney disease (see "How to take Palexia"),
- in patients with pancreatic or biliary tract diseases, including pancreatitis,
- in patients taking drugs with mixed agonist and antagonist properties for opioid receptors "mi" (e.g., pentazocine, nalbuphine) or partial agonists of the opioid receptor "mi" (e.g., buprenorphine),
- in patients with a tendency to epileptic seizures or convulsions, or when taking other drugs that increase the risk of convulsions and may increase the risk of seizures,
This medicine contains tapentadol, which is an opioid. Repeated use of opioid analgesics can lead to reduced efficacy of the medicine (the body getting used to it). It can also lead to addiction and abuse, which can cause life-threatening overdose. If the patient is concerned about becoming addicted to Palexia, it is essential to consult their doctor. Taking the medicine (even in therapeutic doses) can lead to physical dependence, which can cause withdrawal symptoms and recurrence of problems if treatment with this medicine is suddenly stopped.
Palexia can cause physical and psychological dependence. In cases of a tendency to abuse drugs or addiction, treatment should be short-term and under close medical supervision.
Children with obesity should be closely monitored, and the recommended maximum dose should not be exceeded.
Do not give this medicine to children under 2 years of age or with a body weight less than 16 kg.
Sleep apnea
Palexia can cause sleep apnea, such as sleep apnea (pauses in breathing during sleep) and hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to shortness of breath, difficulty maintaining sleep, or excessive daytime sleepiness. If the patient or another person notices such symptoms, they should contact their doctor. The doctor may consider reducing the dose.
Palexia and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
- The risk of side effects increases if you take medicines that can cause seizures (epileptic seizures), such as antidepressants or antipsychotics. The risk of a seizure may increase if you take Palexia at the same time. Your doctor will inform you if taking Palexia is suitable for you.
- Concomitant use of Palexia and sedative medicines, such as benzodiazepine derivatives or benzodiazepine-like medicines [e.g., certain sleeping pills or sedatives (e.g., barbiturates) or painkillers, such as opioids, morphine, and codeine (also used as a cough suppressant), antipsychotics, antihistamines H1, alcohol], increases the risk of drowsiness, breathing difficulties (respiratory depression), and coma, and can be life-threatening. Therefore, concomitant use of such medicines should only be considered when there are no other available treatment options. However, if your doctor prescribes Palexia with sedative medicines, they should limit the dose and duration of concomitant treatment.
Concomitant use of opioids and medicines used to treat epilepsy, neuralgia, or anxiety (gabapentin and pregabalin) increases the risk of opioid overdose, respiratory depression, and can be life-threatening.
Tell your doctor if you are taking gabapentin or pregabalin or any sedative medicine and strictly follow your doctor's dosage instructions. It may be helpful to inform friends or relatives to be aware of the above-mentioned symptoms. If such symptoms occur, contact your doctor.
- If you are taking medicines that affect serotonin levels (e.g., certain medicines used to treat depression), you should consult your doctor before taking Palexia, due to the possibility of "serotonin syndrome". Serotonin syndrome occurs rarely but can be life-threatening. Its symptoms include: uncontrolled, rhythmic muscle contractions, including eye movement muscles, agitation, excessive sweating, trembling, increased reflexes, including increased muscle tension and body temperature above 38°C. If this happens, consult your doctor.
- The concomitant use of Palexia with opioid medicines from the group of mixed agonists/antagonists of the "mi" receptor (e.g., pentazocine, nalbuphine) or partial agonists of the "mi" receptor (e.g., buprenorphine) has not been studied. It is possible that Palexia may not work properly if taken concomitantly with medicines from the above groups. Immediately inform your doctor if you are currently taking any of the above medicines.
- Concomitant use of Palexia with strong inhibitors or inducers (e.g., rifampicin, phenobarbital, St. John's Wort) of the enzymes necessary for the elimination of tapentadol from the body may affect the action of tapentadol or cause side effects, especially when starting or stopping their administration. Tell your doctor about all medicines you are currently taking.
- Palexia should not be used concomitantly with MAO inhibitors (medicines used to treat depression). Tell your doctor if you are taking or have taken these medicines in the last 14 days.
Palexia with food, drink, and alcohol
Do not drink alcohol while taking Palexia, as some side effects, such as drowsiness, may worsen. The medicine can be taken with or without food.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before taking this medicine.
Do not take Palexia:
- during pregnancy, unless your doctor decides to use it. Long-term use of tapentadol during pregnancy may cause withdrawal symptoms in newborns, which can be life-threatening if not recognized and treated by a doctor.
It is not recommended to take Palexia:
- during childbirth, as it may cause dangerous slowing or shallowing of the newborn's breathing (respiratory depression),
- while breastfeeding, as tapentadol may be excreted in breast milk.
Driving and using machines
Palexia can cause drowsiness, dizziness, blurred vision, and affect reaction time.
These symptoms may occur especially at the beginning of treatment with Palexia, after a dose change prescribed by your doctor, or when taking alcohol or sedative medicines. Ask your doctor if driving and using machines are allowed after taking Palexia.
Palexia 20 mg/ml contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per maximum single dose, so it is considered "sodium-free".
Palexia 20 mg/ml contains sodium benzoate
This medicine contains 5.9 mg of sodium benzoate in 5 ml of solution (maximum single dose), which is equivalent to 1.18 mg/ml.
Benzoic acid salt may increase the risk of jaundice (yellowing of the skin and whites of the eyes) in newborns (up to 4 weeks of age).
Palexia 20 mg/ml contains propylene glycol
This medicine contains 10 mg of propylene glycol in 5 ml of solution (maximum single dose), which is equivalent to 2 mg/ml.
3. How to take Palexia
Always take this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
The dosage should be adjusted according to the severity of the pain and the patient's individual sensitivity. The smallest dose that effectively relieves the pain should be used.
Adults
Typically, 50 mg of tapentadol (2.5 ml of oral solution) is taken, 75 mg of tapentadol (3.75 ml of oral solution), or 100 mg of tapentadol (5 ml of oral solution) every 4 to 6 hours.
Total daily doses greater than 700 mg of tapentadol on the first day of therapy and daily doses greater than 600 mg of tapentadol during subsequent days of therapy are not recommended.
Your doctor may prescribe a different, more suitable dose or interval between doses if necessary. If you feel that the effect of the medicine is too strong or too weak, contact your doctor or pharmacist.
Elderly patients
Dose adjustment is not usually necessary in elderly patients (over 65 years).
Elimination of the medicine may be prolonged in this age group, and therefore, your doctor may prescribe a different dosing schedule.
Patients with impaired liver or kidney function
Patients with severe liver impairment should not take this medicine.
In case of moderate liver impairment, your doctor will prescribe a different dosing schedule.
Patients with mild liver impairment do not require dose adjustment.
Patients with severe kidney impairment should not take this medicine.
In case of mild or moderate kidney impairment, dose adjustment is not necessary.
Use in children and adolescents
Palexia should only be given to children in a hospital. Palexia should only be given to children with a body weight over 16 kg.
The dose of Palexia for children over 2 years of age and adolescents under 18 years of age is 1.25 mg/kg every 4 hours.
Always wait 4 hours before giving the next dose. The dose may be reduced due to decreasing acute pain.
The appropriate dose will be determined by your doctor.
Liver and kidney disease (impairment)
Children and adolescents with liver or kidney disease should not take this medicine.
Method of administration
Palexia should be taken orally.
The medicine can be taken on an empty stomach or with food.
The packaging includes a dosing pipette with a connector, which should be used to withdraw the correct amount (volume) of solution from the bottle, corresponding to the prescribed dose of tapentadol.
Instructions for opening the bottle and using the dosing pipette
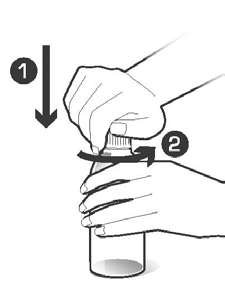
The bottle has a child-resistant closure. To open the bottle, press the cap and turn it counterclockwise (Figure 1).
Remove the cap and the protective foil from the top of the bottle. If the protective foil is damaged, do not use the product and contact your pharmacist.
Figure 1
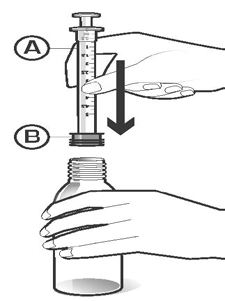
Place the bottle on a hard, flat surface. Open the plastic bag containing the dosing pipette with the connector by tearing the perforated part of the bag and remove the dosing pipette (A) with the attached connector (B). Screw the connector with the dosing pipette tightly into the bottle neck (Figure 2).
Figure 2
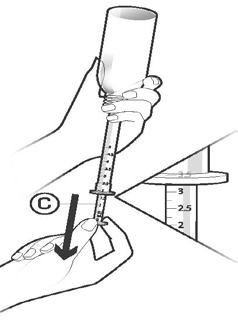
To fill the dosing pipette, turn the bottle upside down.
Holding the dosing pipette, gently pull the plunger (C) down to the line marking the dose prescribed by your doctor (see "How to take Palexia"). Do not removethe dosing pipette during this operation! (Figure 3).
Figure 3
Turn the bottle uprightand carefully remove the dosing pipette from the bottle. After removing the dosing pipette, carefully check if the correct amount of solution has been withdrawn. The connector (B), which was previously attached to the dosing pipette, should now remain in the bottle (Figure 4).
Figure 4
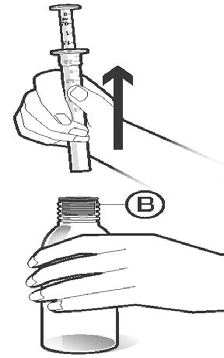
Figure 4
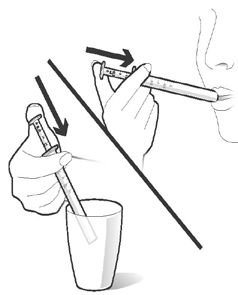
Take the medicine by placing the dosing pipette in your mouth and gently pressing the plunger. Press the plunger all the way down to ensure that the entire solution is taken. The medicine can be diluted in a glass of water or another non-alcoholic beverage before taking it. In this case, drink the entire glass of beverage to ensure that the correct dose of the medicine has been taken (Figure 5).
Figure 5
Leave the connector in the bottle, close the bottle tightly, and store it upright.
After each use, wash the dosing pipette with water and let it dry.
Before taking the medicine again, place the dosing pipette in the connector in the bottle neck and follow the above instructions.
Duration of treatment
Do not take the medicine for longer than your doctor recommends. In children, the treatment duration should not exceed 3 days.
Taking a higher dose of Palexia than recommended
After taking very high doses, the following symptoms may occur:
- constricted pupils to the size of a pinhead, vomiting, decreased blood pressure, rapid heartbeat, collapse, impaired consciousness or coma (deep state of loss of consciousness), seizures, dangerously slow or shallow breathing or respiratory arrest. In such cases, seek medical help immediately!
Missing a dose of Palexia
If you miss a dose, your pain is likely to return.
Do not take a double dose to make up for the missed dose.
Return to your previous dosing schedule.
Stopping treatment with Palexia
If you stop taking Palexia or discontinue treatment before it is completed, your pain is likely to return.
Contact your doctor before stopping the medicine.
Generally, no side effects are observed after stopping the medicine; however, in rare cases, patients taking the medicine for some time and stopping it abruptly may experience general malaise.
The following symptoms may occur:
- restlessness, tearfulness, runny nose, yawning, sweating, chills, muscle pain, and dilated pupils,
- irritability, anxiety, back pain, joint pain, weakness, abdominal cramps, difficulty sleeping, nausea, loss of appetite, vomiting, diarrhea, increased blood pressure, respiratory rate, and heart rate. If you experience any of these symptoms, consult your doctor immediately.
Do not stop taking Palexia abruptly, unless your doctor advises you to do so.
Your doctor will inform you how to stop taking the medicine. Stopping the medicine may involve gradually reducing the dose.
If you have any further doubts about taking this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Palexia can cause side effects, although not everybody gets them.
Important side effects or symptoms to watch out for and what to do if they occur:
This medicine can cause allergic reactions. Symptoms may include wheezing, difficulty breathing, swelling of the eyelids, face, or lips, rash, or itching, especially affecting the whole body.
Another serious side effect is excessive drowsiness and respiratory depression. This occurs most commonly in elderly and weakened patients.
If any of these important side effects affect you, contact your doctor immediately.
Other side effects that may occur:
Very common(may affect more than 1 in 10 people): nausea, vomiting, dizziness, drowsiness, headache.
Common(may affect up to 1 in 10 people): loss of appetite, anxiety, confusion, hallucinations, sleep disturbances, unusual dreams, tremors, flushing, constipation, diarrhea, indigestion, dry mouth, itching, excessive sweating, rash, muscle spasms, feeling of weakness, fatigue, feeling of temperature change.
Uncommon(may affect up to 1 in 100 people): low mood, disorientation, agitation (irritation), nervousness, restlessness, euphoric mood, concentration disorders, memory impairment, feeling of impending fainting, excessive sedation, balance disorders, speech disorders, tingling, abnormal skin sensations (e.g., burning, prickling), muscle spasms, vision disturbances, rapid heartbeat, palpitations, decreased blood pressure, dangerously slow or shallow breathing (respiratory depression), decreased oxygen saturation, shortness of breath, discomfort in the abdominal cavity, hives, feeling of heaviness, difficulty urinating, frequent urination, withdrawal syndrome (see "Stopping treatment with Palexia"), water retention (edema), feeling of abnormality, feeling of intoxication, irritability, feeling of relaxation.
Rare(may affect up to 1 in 1000 people): allergic reactions to the medicine (including skin swelling, hives, and in severe cases, difficulty breathing, decreased blood pressure, collapse, or shock), thinking disorders, seizures, decreased level of consciousness, coordination disorders, slow heartbeat, impaired gastric emptying.
Frequency not known: delirium.
Generally, the likelihood of suicidal thoughts and behaviors is higher in patients with chronic pain. Additionally, medicines used to treat depression (which affect the neurotransmitter system in the brain) may increase this risk, especially at the beginning of treatment. Although tapentadol also affects neurotransmitters, data from human use have not provided evidence of an increased risk.
No other side effects have been observed in children and adolescents.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to:
Department for Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products,
Jerozolimskie Avenue 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Palexia
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and bottle. The expiry date refers to the last day of the month.
Unopened: no special precautions for storage are necessary.
After opening the bottle, the solution should not be used for more than 6 weeks.
Store in an upright position.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Palexia contains
- -Active substanceof Palexia is tapentadol.
1 ml of Palexia, 20 mg/ml, oral solution, contains 20 mg of tapentadol (as tapentadol hydrochloride).
- -Otheringredients are: Sodium benzoate (E 211) Citric acid monohydrate Sucralose (E 955) Raspberry flavor, containing propylene glycol (E 1520) Sodium hydroxide (for pH adjustment) Purified water
What Palexia looks like and contents of the pack
Palexia is a clear, colorless oral solution.
Palexia, 20 mg/ml, oral solution, is available in HDPE bottles with a child-resistant closure, containing 100 ml or 200 ml of solution, with a 5 ml dosing pipette with 0.1 ml graduations and a connector made of LDPE, attached to the dosing pipette, in a cardboard box. Additionally, a scale on the right side of the box shows single doses intended for adults.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Grünenthal GmbH
Zieglerstrasse 6
52078 Aachen
Germany
For more information about this medicine, contact the representative of the marketing authorization holder:
Stada Pharm Sp. z o.o.
Krakowiaków 44
02-255 Warsaw
Tel. +48 22 737 79 20
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
PALEXIA: Austria, Belgium, Cyprus, Croatia, Czech Republic, Netherlands, Germany, Greece, Ireland, Italy, Luxembourg, Norway, Poland, Portugal, Slovakia, Slovenia, Spain, United Kingdom (Northern Ireland).
Date of last revision of the leaflet:07/2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGruenenthal GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
Talk to a doctor online
Need help understanding this medicine or your symptoms? Online doctors can answer your questions and offer guidance.














