
Oxirin Apteo Med
Ask a doctor about a prescription for Oxirin Apteo Med

How to use Oxirin Apteo Med
Package Leaflet: Information for the User
Oxyrin Apteo Med
0.5 mg/ml
nasal spray, solution
Oxymetazoline hydrochloride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient or as advised by a doctor or pharmacist.
- The package leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any not listed in this leaflet, the doctor or pharmacist, or nurse should be informed. See section 4.
- A doctor should be contacted if there is no improvement or the patient feels worse after 7 days.
Table of Contents of the Package Leaflet:
- 1. What is Oxyrin Apteo Med and what is it used for
- 2. Important information before taking Oxyrin Apteo Med
- 3. How to take Oxyrin Apteo Med
- 4. Possible side effects
- 5. How to store Oxyrin Apteo Med
- 6. Package contents and other information
1. What is Oxyrin Apteo Med and what is it used for
Oxyrin Apteo Med belongs to a group of medicines called sympathomimetics, which act locally to constrict blood vessels and decongest the nose.
The medicine is indicated for local and temporary relief of nasal congestion caused by rhinitis.
A doctor should be contacted if there is no improvement or the patient feels worse after 7 days.
2. Important information before taking Oxyrin Apteo Med
When not to use Oxyrin Apteo Med
- if the patient is allergic to oxymetazoline or any of the other ingredients of this medicine (listed in section 6);
- if the patient is taking or has taken within the last 14 days antidepressant medicines (used to treat depression);
- if the patient is taking other sympathomimetic decongestant medicines;
- if the patient has high fluid pressure in the eye (narrow-angle glaucoma);
- if the patient has recently undergone neurosurgical operations (transsphenoidal removal of the pituitary gland or other surgery involving exposure of the dura mater);
- if the patient has heart disease or circulatory disorders;
- if the patient has dry rhinitis with crust formation (atrophic rhinitis).
Oxyrin Apteo Med should not be used in children under 12 years of age.
Warnings and precautions
Before starting to use Oxyrin Apteo Med, the patient should discuss it with a doctor or pharmacist:
- if the patient has high blood sugar levels (diabetes);
- if the patient has high blood pressure (hypertension);
- if the patient has prostate problems (enlarged prostate);
- if the patient has thyroid problems (hyperthyroidism);
- if the patient has a tumor of the adrenal gland (pheochromocytoma);
- if the patient is taking adrenergic bronchodilator medicines (for asthma), phenothiazines (sedatives), or methyldopa (to lower blood pressure);
- if the patient experiences insomnia (very rare cases), the medicine should be avoided in the late evening or at night.
Using the same bottle of nasal spray by more than one person may lead to the spread of infection.
Children and adolescents
Oxyrin Apteo Med should not be used in children under 12 years of age. Children may be more susceptible to side effects.
Oxyrin Apteo Med and other medicines
The patient should tell the doctor or pharmacist about all medicines being taken, including those obtained without a prescription, homeopathic medicines, herbal products, and other health-related products, as it may be necessary to stop treatment or adjust the dose of some of them.
Some medicines may interact with Oxyrin Apteo Med, and in such cases, the dose may need to be changed or treatment stopped. It is essential to inform the doctor or pharmacist if the patient is taking any of the following medicines: antidepressant medicines (monoamine oxidase inhibitors (MAOIs) or tricyclic antidepressants), phenothiazine derivatives (sedatives), anti-asthmatic, antihypertensive, or methyldopa (to lower blood pressure), bromocriptine (for Parkinson's disease), cardiac glycosides (digoxin, for heart rate control), or ergot alkaloids (for migraine headaches).
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult a doctor or pharmacist before using this medicine.
Pregnancy
Oxymetazoline should not be used during pregnancy.
Breastfeeding
The medicine should not be used during breastfeeding without consulting a doctor, as it may pass into breast milk.
Driving and using machines
Although it is not expected that the medicine will affect the ability to drive or use machines, if the patient feels drowsy or dizzy, they should not drive or operate machinery.
Oxyrin Apteo Med contains benzalkonium chloride
Oxyrin Apteo Med contains 0.2 mg of benzalkonium chloride per ml of nasal spray.
Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time.
3. How to take Oxyrin Apteo Med
This medicine should always be used exactly as described in the package leaflet for the patient or as advised by a doctor or pharmacist. If in doubt, the patient should consult a doctor or pharmacist.
Adults and children over 12 years of age
The recommended dose is one spray into each nostril, up to two times a day.
The medicine should not be used for more than 7 days without consulting a doctor.
One dose of the nasal spray before bedtime should ensure nasal patency throughout the night.
Using the medicine in children
Oxyrin Apteo Med should not be used in children under 12 years of age. Children may be more susceptible to side effects.
Instructions for proper use of the medicine
Before using the medicine for the first time, the spray should be loaded. To do this, the patient should hold the bottle away from themselves and press the pump several times, directing it downwards, until the spray is released.
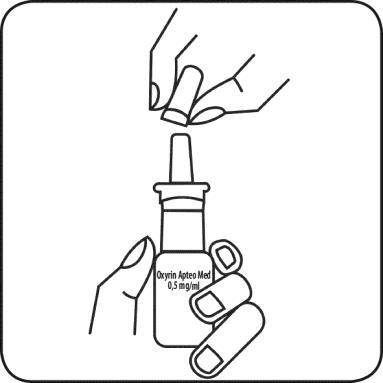
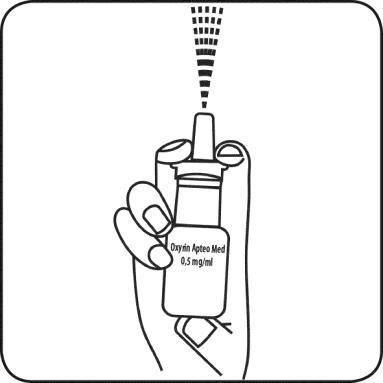
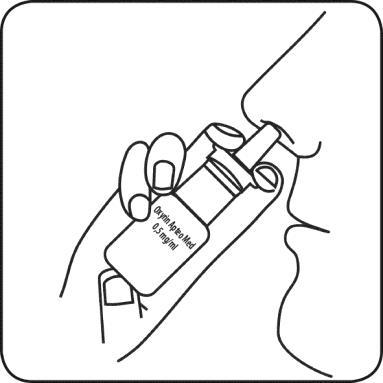
- 1. Remove the protective cap from the bottle
- 2. Hold the bottle and place the middle and index fingers in the upper and lower parts, and hold the thumb
- 3. Insert the applicator tip into the nostril and press
Before using the medicine, the nose should be cleaned.
After each use and before closing the bottle, the applicator should be wiped with a clean, damp cloth.
The doctor or pharmacist should be informed if the patient feels that the effect of the medicine is too strong or too weak.
Using a higher dose of Oxyrin Apteo Med than recommended
In case of using too high a dose or continuous use, or accidental ingestion of Oxyrin Apteo Med, the patient may experience headaches, tremors, sleep disturbances, excessive sweating, palpitations, and nervousness. If such symptoms occur, the dose should be reduced, and a doctor or pharmacist should be consulted if the patient is unsure of the correct dose.
In case of overdose or accidental ingestion, a doctor, pharmacist, or the nearest hospital emergency department should be informed immediately.
Missing a dose of Oxyrin Apteo Med
A double dose of the medicine should not be taken to make up for a missed dose.
If there are any further doubts about using this medicine, a doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, Oxyrin Apteo Med can cause side effects, although not everybody gets them.
Common(may affect up to 1 in 10 people): burning, dryness, and (or) irritation of the nasal mucosa or sneezing. Prolonged or excessive use of the medicine may cause rebound congestion of the nasal mucosa.
Rare(may affect up to 1 in 1000 people): anxiety, fatigue, irritability, sleep disturbances in children, rapid heartbeat, palpitations, increased blood pressure, feeling of a blocked nose, swelling of the nasal mucosa, headache, nausea, redness, rash, and vision disturbances.
Reporting side effects
If any side effects occur, including any not listed in this leaflet, the doctor or pharmacist, or nurse should be informed. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: (22) 49 21 301, fax: (22) 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Oxyrin Apteo Med
Do not store above 30°C.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP. The expiry date refers to the last day of the month. The medicine should be used within 30 days of first opening.
Medicines should not be disposed of via wastewater or household waste. A pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Oxyrin Apteo Med contains
- The active substance is oxymetazoline hydrochloride 0.5 mg/ml.
- The other ingredients are: benzalkonium chloride, anhydrous disodium phosphate, sodium dihydrogen phosphate dihydrate, glycine, non-crystallizing sorbitol, purified water.
What Oxyrin Apteo Med looks like and contents of the pack
Oxyrin Apteo Med is a nasal spray solution in a polyethylene bottle with a pump spray and cap. Each bottle contains 15 ml of solution.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Synoptis Pharma Sp. z.o.o.
ul. Krakowiaków 65
02-255 Warsaw
Manufacturer:
ITALFARMACO, S.A.
San Rafael, 3
28108 Alcobendas (Madrid)
Spain
Date of last revision of the leaflet:August 2021
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterItalfarmaco S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Oxirin Apteo MedDosage form: Aerosol, 0.25 mg/mlActive substance: oxymetazolineManufacturer: Lomapharm GmbHPrescription not requiredDosage form: Aerosol, 0.5 mg/mlActive substance: oxymetazolineManufacturer: Lomapharm GmbHPrescription not requiredDosage form: Aerosol, 0.5 mg/ml (0.05%)Active substance: oxymetazolinePrescription not required
Alternatives to Oxirin Apteo Med in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Oxirin Apteo Med in Ukraine
Alternative to Oxirin Apteo Med in Spain
Online doctors for Oxirin Apteo Med
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Oxirin Apteo Med – subject to medical assessment and local rules.








