
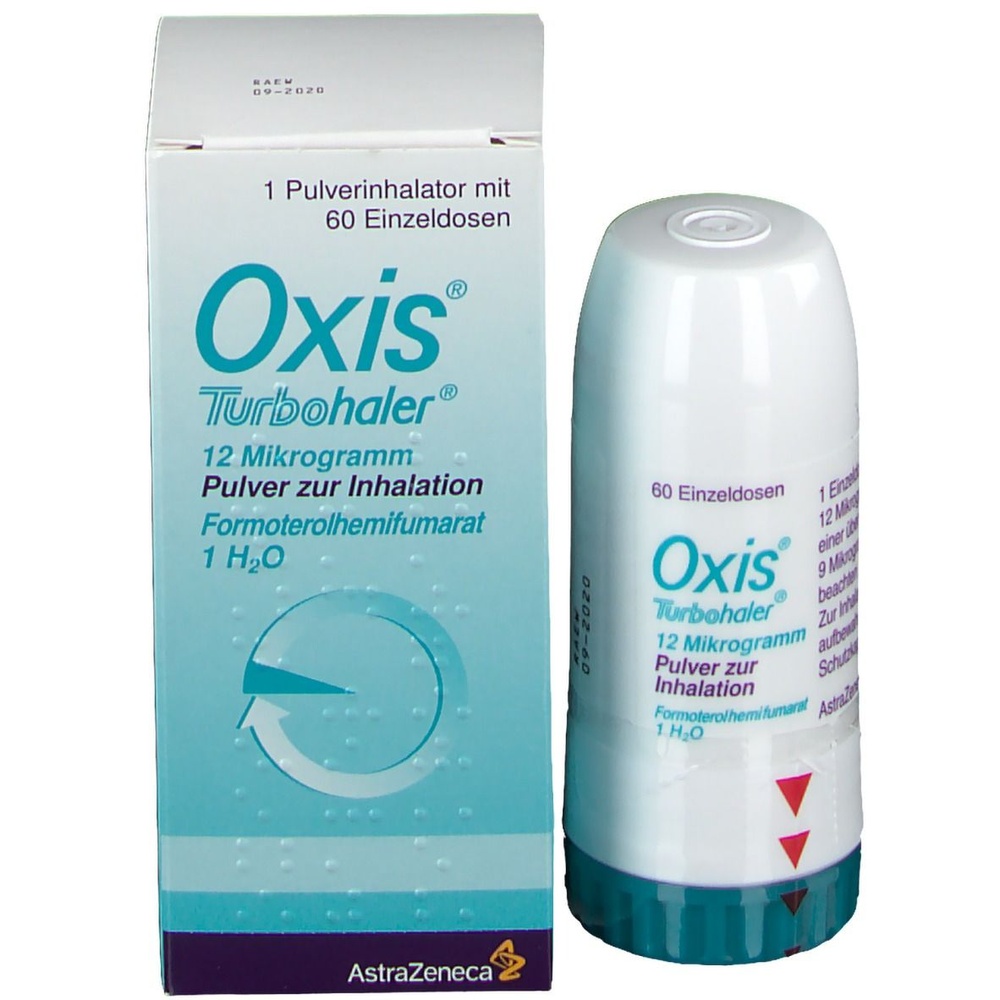
Oxis Turbuhaler

Ask a doctor about a prescription for Oxis Turbuhaler

How to use Oxis Turbuhaler
Leaflet attached to the packaging: patient information
Oxis Turbuhaler, 9 micrograms/dose, inhalation powder
Formoterol fumarate dihydrate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Oxis Turbuhaler and what is it used for
- 2. Important information before using Oxis Turbuhaler
- 3. How to use Oxis Turbuhaler
- 4. Possible side effects
- 5. How to store Oxis Turbuhaler
- 6. Contents of the pack and other information
1. What is Oxis Turbuhaler and what is it used for
Formoterol, the active substance of Oxis Turbuhaler, is a selective β2-agonist.
Its action is based on the relaxation of smooth muscles in the bronchi. This makes breathing easier.
When inhaling through the mouthpiece of the inhaler, the medicine gets into the lungs. The action of the medicine
starts within 1 to 3 minutes after inhalation and lasts for 12 hours.
Oxis Turbuhaler is indicated for the maintenance treatment, in combination with inhaled glucocorticosteroids, of symptoms of airway obstruction and for the prevention of symptoms caused by exercise in patients with asthma, if treatment with glucocorticosteroids is not sufficient.
Oxis Turbuhaler is also indicated for the treatment of symptoms of airway obstruction in patients with chronic obstructive pulmonary disease (COPD).
2. Important information before using Oxis Turbuhaler
When not to use Oxis Turbuhaler
- if the patient is allergic (hypersensitive) to formoterol or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Oxis Turbuhaler is not intended for the initiation of asthma treatment, as it is not sufficient for this purpose.
Use of Oxis Turbuhaler should not be started during an acute, severe asthma exacerbation or during worsening of existing exacerbation. During treatment, exacerbations and serious side effects related to the course of asthma may occur.
If there is no improvement in symptoms after starting treatment or if exacerbation occurs, the patient should continue to take the medicine and consult the doctor who prescribed it.
The patient should inform the doctor about any worrying reactions that have occurred in the past after using formoterol or lactose, or after taking other medicines. In some cases, Oxis Turbuhaler should be used with caution. The patient should especially inform the doctor about heart disease, diabetes, thyroid function disorders (hyperthyroidism), pheochromocytoma, hypertension, aneurysm, and decreased potassium levels in the blood.
If bronchospasm occurs after using the medicine, the patient should stop using it immediately and consult a doctor.
Oxis Turbuhaler should be used in the treatment of asthma together with glucocorticosteroids.
The patient should continue to take glucocorticosteroids after starting Oxis Turbuhaler, even if symptoms improve. In case of an acute asthma attack, additional doses of Oxis Turbuhaler can be used. However, if it is necessary to use additional doses of Oxis Turbuhaler more frequently than usual to relieve breathing difficulties, the patient should consult their doctor immediately. In such a case, additional treatment may be necessary.
In patients with diabetes, the doctor may recommend monitoring blood glucose levels during the initial treatment period.
In patients with severe asthma who are taking other medicines, the doctor may recommend monitoring potassium levels in the blood.
The patient should strictly follow the doctor's recommendations and not change them without prior consultation.
Children
Oxis Turbuhaler should not be used in children under 6 years of age due to insufficient data on the use of the medicine in this age group.
Oxis Turbuhaler and other medicines
Other medicines used at the same time may affect the action of Oxis Turbuhaler.
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those available without a prescription.
Especially, the patient should inform the doctor if they are taking any of the following medicines:
- xanthine derivatives (e.g., theophylline, aminophylline) - medicines used to treat asthma or other chronic obstructive pulmonary diseases,
- steroids (medicines used to treat asthma and other inflammatory diseases),
- diuretics (e.g., furosemide),
- quinidine, disopyramide, or procainamide (medicines used to treat heart rhythm disorders),
- phenothiazines (e.g., chlorpromazine), used to treat mental disorders,
- antihistamines (e.g., terfenadine),
- monoamine oxidase inhibitors (MAOIs), used to treat depression,
- levodopa (used to treat Parkinson's disease),
- levothyroxine (used to treat hypothyroidism),
- oxytocin (used to induce uterine contractions),
- tricyclic antidepressants (used to treat depression),
- β-adrenergic receptor blockers (such as atenolol or propranolol, used to treat high blood pressure), also in the form of eye drops (such as timolol used in glaucoma),
- anticholinergic medicines (such as tiotropium or ipratropium bromide) may enhance the bronchodilatory effect of Oxis Turbuhaler. The patient should inform their doctor about the use of these medicines if they are to undergo general anesthesia or dental procedures.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor before using this medicine.
If a woman using Oxis Turbuhaler becomes pregnant, she should inform her doctor as soon as possible.
It is not known whether formoterol passes into breast milk, so Oxis Turbuhaler should not be used by breastfeeding mothers.
Driving and using machines
Using Oxis Turbuhaler does not affect the ability to drive or use machines.
Oxis Turbuhaler contains lactose
If the patient has previously been diagnosed with intolerance to some sugars, they should consult their doctor before taking the medicine.
One dose of Oxis Turbuhaler 9 micrograms/dose contains 891 micrograms of lactose monohydrate, which is usually of no clinical significance in patients with lactose intolerance.
Lactose monohydrate contains small amounts of milk proteins. These may cause allergic reactions.
3. How to use Oxis Turbuhaler
Oxis Turbuhaler should always be used as directed by the doctor. If there are any doubts, the patient should consult their doctor. The dosage of Oxis Turbuhaler is determined individually.
Asthma
Oxis Turbuhaler can be used in patients with asthma once or twice a day (maintenance doses) or as needed to relieve breathing difficulties.
Adults over 18 years of age
As-needed use
1 inhalation to relieve breathing difficulties.
Maintenance dose
1 inhalation once or twice a day. In some patients, it may be necessary to use 2 inhalations once or twice a day.
Prevention of exercise-induced bronchospasm
1 inhalation before exercise.
The maximum daily maintenance dose of Oxis Turbuhaler should not exceed 4 inhalations, but up to 6 inhalations per day may be used if necessary.
The maximum single dose is 3 inhalations.
Children aged 6 and over
As-needed use
1 inhalation to relieve breathing difficulties.
Maintenance dose
1 inhalation once or twice a day.
Prevention of exercise-induced bronchospasm
1 inhalation before exercise.
The maximum daily maintenance dose of Oxis Turbuhaler should not exceed 2 inhalations, but up to 4 inhalations per day may be used if necessary.
The maximum single dose is 1 inhalation.
The doctor will monitor the patient's asthma and adjust the dose of Oxis Turbuhaler, gradually reducing it to the smallest dose that controls the asthma.
However, the patient should not change the dose of the medicine without prior consultation with their doctor.
Chronic obstructive pulmonary disease (COPD)
Adults over 18 years of age
Maintenance dose
1 inhalation once or twice a day.
The maximum daily maintenance dose should not exceed 2 inhalations.
In patients treated with Oxis Turbuhaler, additional doses (inhalations) may be used as needed, in addition to the maintenance treatment, to relieve symptoms.
The maximum daily maintenance dose is 4 inhalations (including those used regularly).
The maximum single dose is 2 inhalations.
Special patient groups
No dose adjustment of Oxis Turbuhaler is necessary in elderly patients, patients with liver or kidney function disorders, if the recommended doses are used.
Note:
The need to use additional doses (inhalations), in addition to the maintenance treatment, more than 2 times a week indicates that the treatment is insufficient.
In such a case, the patient should consult their doctor as soon as possible.
Before starting treatment with Oxis Turbuhaler, the patient should read the “Instructions for use of Oxis Turbuhaler”below.
Due to the very small amount of powdered active substance in the delivered dose, the patient may not feel the taste of the medicine after inhalation. However, if the patient follows the instructions, they can be sure that the prescribed dose has been taken and the medicine is in the lungs.
Instructions for use of Oxis Turbuhaler
Preparing the Oxis Turbuhaler inhaler for first use
Before firstuse of a newOxis Turbuhaler inhaler, the patient should prepare it for use as follows:
- Remove the cap from the inhaler. When removing the cap, a characteristic rattling sound may be heard. This is caused by the desiccant moving inside the inhaler.
- Hold the Oxis Turbuhaler inhaler upright with the turquoise turning grip downwards.
- Turn the turquoise turning grip as far as it will go in one direction, then turn it as far as it will go in the opposite direction (the direction in which the turning grip is started does not matter). A characteristic click will be heard.
- Only before the first use of the inhaler, the above step should be repeated, turning the turquoise turning grip in both directions.
- The Oxis Turbuhaler inhaler is now ready for use.
Using the inhaler
To perform an inhalation, the patient should follow the instructions below each time.
- 1. Remove the cap from the inhaler. When removing the cap, a characteristic rattling sound may be heard.
- 2. Hold the Turbuhaler inhaler in a vertical position, with the turquoise turning grip downwards.
- 3. When loading a dose, do not hold the inhaler by the mouthpiece. To load a dose, turn the turquoise turning grip as far as it will go in one direction, then turn it as far as it will go in the opposite direction (the direction in which the turning grip is started does not matter). A characteristic click will be heard. The Turbuhaler inhaler is now loaded and ready for use. The patient should only load a dose immediately before use.
- 4. Move the Turbuhaler inhaler away from the mouth and exhale. Do not exhale through the mouthpiece of the inhaler.
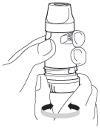
- 5. Place the mouthpiece of the inhaler gently between the teeth. Close the lips around the mouthpiece and take a slow, deep, and long breath in through the mouth. Do not chew or bite the mouthpiece. Do not use a damaged inhaler or an inhaler without a mouthpiece.
- 6. Before exhaling, remove the inhaler from the mouth. Then exhale. The amount of medicine in one inhalation is very small, so the patient may not feel the taste of the medicine after inhalation. However, if the patient follows the instructions, they can be sure that the dose of the medicine has been taken and the medicine is in the lungs.
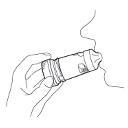
- 7. If a second inhalation is prescribed, the patient should repeat the steps described in points 2 to 6.
- 8. After use, put the cap back on the inhaler and close it tightly.
- 9. Do not remove the mouthpiece, as it is connected to the Turbuhaler inhaler and cannot be removed. The mouthpiece can be rotated, but it should not be turned unnecessarily. Do not use a Turbuhaler inhaler that has been damaged or if the mouthpiece is not attached to the inhaler.
As with all inhalers, caregivers should ensure that children who have been prescribed Oxis Turbuhaler use the correct inhalation technique, as described above.
Cleaning the Turbuhaler inhaler
The patient should wipe the outside of the mouthpiece with a dry cloth once a week. Do not use water or other liquids to clean the inhaler.
When to start using a new inhaler
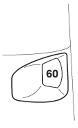
- The dose counter window shows how many doses (inhalations) are left in the Turbuhaler inhaler; it starts at 60, because a full inhaler contains 60 doses of the medicine.
- The dose counter scale is marked at intervals of 10 inhalations, so the counter does not show a change after a single dose of the medicine.
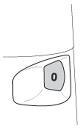
- The appearance of a red mark on the edge of the dose counter window indicates that there are about 20 doses of the medicine left in the inhaler. During the administration of the last 10 doses of the medicine, the background of the dose counter window is red. When the number 0 on the red background is in the middle of the dose counter window, it means that the patient should start using a new Turbuhaler inhaler.
Note:
- After the inhaler is empty, the turning grip will still turn and make a characteristic click.
- The sound heard when shaking the Turbuhaler inhaler is made by the desiccant, not the medicine. Therefore, the contents of the Turbuhaler inhaler cannot be assessed based on the sound made by the inhaler when it is shaken.
- If more than one dose is loaded by mistake, the patient will still receive only one dose of the medicine. However, all loaded doses will be recorded in the dose counter.
Using a higher than recommended dose of Oxis Turbuhaler
Overdose may cause the following symptoms: muscle tremors, headaches, rapid heartbeat, dizziness, drowsiness.
If the patient has taken a higher dose of the medicine than recommended, they should immediately consult their doctor or go to the hospital.
Missing a dose of Oxis Turbuhaler
Oxis Turbuhaler inhalation powder should be used as directed by the doctor and only the prescribed number of doses should be taken. The patient should not take a double dose to make up for a missed dose.
Stopping treatment with Oxis Turbuhaler
If the patient has any further doubts about using the medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Oxis Turbuhaler can cause side effects, although not everybody gets them.
As with other inhaled medicines, very rarely (less than 1 in 10,000 patients), paradoxical bronchospasm may occur. If bronchospasm occurs after using the medicine, the patient should stop using it and consult their doctor immediately.
Common side effects (in 1 to 10 patients in 100)
- headaches,
- tremors,
- dizziness,
- nausea,
- muscle spasms.
Uncommon side effects (in 1 to 10 patients in 1,000)
- hypersensitivity reactions (e.g., bronchospasm, rash, urticaria, itching),
- decreased potassium levels in the blood,
- increased glucose levels in the blood,
- rapid heartbeat,
- palpitations,
- arrhythmias (e.g., atrial fibrillation, supraventricular tachycardia, and extrasystoles),
- changes in blood pressure,
- symptoms of angina pectoris (pain and feeling of pressure in the chest),
- sleep disturbances,
- taste disturbances.
Rare side effects (in 1 to 10 patients in 10,000)
- agitation,
- restlessness.
Very rare side effects (less than 1 in 10,000 patients)
- abnormalities in the ECG recording. During treatment with β2-agonists, there may be an increase in insulin, free fatty acids, glycerol, and ketone bodies in the blood. In some patients, other side effects may occur during treatment with Oxis Turbuhaler.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
Side effects can also be reported to the marketing authorization holder.
5. How to store Oxis Turbuhaler
Store out of sight and reach of children.
There are no special precautions for storage.
The inhaler should be stored with the cap tightly closed.
Do not use Oxis Turbuhaler after the expiry date stated on the carton and the label on the Turbuhaler.
The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Oxis Turbuhaler contains
- The active substance of Oxis Turbuhaler is formoterol. One delivered dose contains 9 micrograms of formoterol fumarate dihydrate.
- The other ingredient of the medicine is lactose monohydrate (which contains milk proteins). See section 2 of the leaflet: Oxis Turbuhaler contains lactose.
What Oxis Turbuhaler looks like and contents of the pack
The carton contains one inhaler, which holds 60 doses of the medicine.
Each inhaler is additionally labeled with:
“60 DOSES FORMOTEROL 9 μg/DOSE
LOT/LOTE ZM 1234
EXP/CAD MM-YYYY”,
which means:
“60 DOSES OF FORMOTEROL 9 μg/DOSE
SERIAL NUMBER ZM 1234
EXPIRY DATE: MM-YYYY”
Marketing authorization holder and manufacturer
Marketing authorization holder
AstraZeneca AB
S-151 85 Sodertalje
Sweden
Manufacturer
AstraZeneca AB
Forskargatan 18
SE-151 36 Södertälje
Sweden
To obtain more detailed information, the patient should contact the representative of the marketing authorization holder:
AstraZeneca Pharma Poland Sp. z o.o.
ul. Postępu 14
02-676 Warsaw
tel: +48 22 245 73 00
fax: +48 22 485 30 07
Date of last revision of the leaflet:June 2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAstraZeneca AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Oxis TurbuhalerDosage form: Aerosol, 12 mcg/measured doseActive substance: formoterolManufacturer: Chiesi Farmaceutici S.p.A. Chiesi Pharmaceuticals GmbHPrescription requiredDosage form: Powder, 12 mcgActive substance: formoterolPrescription requiredDosage form: Powder, 12 mcg/inh. doseActive substance: formoterolPrescription required
Alternatives to Oxis Turbuhaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Oxis Turbuhaler in Ukraine
Alternative to Oxis Turbuhaler in Spain
Online doctors for Oxis Turbuhaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Oxis Turbuhaler – subject to medical assessment and local rules.














