

Otrivin Menthol

Ask a doctor about a prescription for Otrivin Menthol

How to use Otrivin Menthol
Package Leaflet: Information for the User
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Otrivin Menthol, 1 mg/ml, nasal spray, solution
Xylometazoline hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this leaflet for the patient or as directed by a doctor or pharmacist.
- The leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist or nurse should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
- If there is no improvement after 10 days or the patient feels worse, they should contact their doctor.
Table of Contents of the Leaflet:
- 1. What is Otrivin Menthol and what is it used for
- 2. Important information before using Otrivin Menthol
- 3. How to use Otrivin Menthol
- 4. Possible side effects
- 5. How to store Otrivin Menthol
- 6. Contents of the pack and other information
1. What is Otrivin Menthol and what is it used for
The active substance of Otrivin Menthol is xylometazoline hydrochloride.
Otrivin Menthol is a locally acting decongestant that reduces nasal congestion and swelling of the nasal mucosa. It unblocks the nose, making breathing easier.
The medicine also contains excipients such as menthol and eucalyptol, which give a cooling sensation on the mucous membranes.
The effect of Otrivin Menthol starts within 2 minutes and lasts for up to 12 hours.
Otrivin Menthol is indicated for use in adults and adolescents over 12 years of age.
Otrivin Menthol is used for excessive nasal congestion in the common cold, hay fever, allergic rhinitis, and sinusitis.
By reducing nasal congestion, the medicine can also be used as an adjunct in the treatment of middle ear infections. Otrivin Menthol facilitates nasal examination (examination of the nasal cavity).
Otrivin Menthol does not contain preservatives.
2. Important information before using Otrivin Menthol
When not to use Otrivin Menthol
- if the patient is allergic to xylometazoline hydrochloride or any of the other ingredients of this medicine (listed in section 6),
- if the patient has undergone surgery through the nose, e.g., removal of the pituitary gland or an operation
involving exposure of the dura mater (brain surgery performed through the nose or mouth),
- if the patient has narrow-angle glaucoma (increased eye pressure),
- if the patient's nasal mucosa is very dry (atrophic rhinitis or dry rhinitis),
- in children under 12 years of age.
Warnings and precautions
Before using the medicine, the patient should consult a doctor or pharmacist if they have:
- high blood pressure,
- heart disease (e.g., long QT syndrome),
- hyperthyroidism,
- diabetes,
- enlarged prostate,
- a benign tumor of the adrenal gland that produces large amounts of adrenaline and noradrenaline (pheochromocytoma),
- are taking certain antidepressants known as monoamine oxidase inhibitors (MAOIs) or have taken them in the last 2 weeks,
- are taking tricyclic or tetracyclic antidepressants,
- like other nasal decongestants, Otrivin Menthol may cause insomnia, dizziness, tremors, heart rhythm disturbances, and increased blood pressure in sensitive patients. If such symptoms occur, the patient should consult a doctor,
- like other nasal decongestants, Otrivin Menthol should not be used for more than 10 consecutive days. If symptoms persist, the patient should consult a doctor. Using the medicine for a longer period than 10 days or in higher doses than recommended may worsen symptoms or cause them to recur.
- Otrivin Menthol should not be used in the eyes or mouth.
- A higher dose than recommended should not be used, especially in children and the elderly.
Children and adolescents
Otrivin Menthol should not be used in children under 12 years of age.
Otrivin Menthol and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Otrivin Menthol should not be used in combination with certain medicines used to treat depression. These include:
- monoamine oxidase inhibitors (MAOIs): Otrivin Menthol should not be used if the patient is taking MAOIs or has taken them in the last 14 days,
- tricyclic or tetracyclic antidepressants.
If the patient has taken any of these medicines before using Otrivin Menthol, they should consult a doctor.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor before using this medicine.
Otrivin Menthol should not be used during pregnancy.
During breastfeeding, Otrivin Menthol can be used only after consulting a doctor.
Driving and using machines
Otrivin Menthol, when used as directed, does not affect or has a negligible effect on the ability to drive or use machines.
Otrivin Menthol contains macrogolglycerol hydroxystearate (hydrogenated polyoxyethylene castor oil)
This medicine contains 2.75 mg/ml of macrogolglycerol hydroxystearate (hydrogenated polyoxyethylene castor oil). The medicine may cause skin reactions.
3. How to use Otrivin Menthol
This medicine should always be used exactly as described in this leaflet for the patient or as directed by a doctor or pharmacist or nurse. If in doubt, the patient should consult a doctor or pharmacist or nurse.
Otrivin Menthol should not be used in children under 12 years of age.
Adults and adolescents over 12 years of age:
1 dose of Otrivin Menthol nasal spray into each nostril up to 3 times a day. It is recommended to take the last dose of the medicine directly before going to bed.
Do not use more than 3 times a day into each nostril.
Otrivin Menthol should not be used for more than 10 consecutive days.
Method of administration
The medicine is intended for nasal use.
Be careful not to spray the aerosol into the eyes.
- 1. Clean the nose.
- 2. Remove the protective cap.
- 3. Do not cut the tip of the applicator.
- 4. Before the first administration, press the pump 4 times. The pump will be ready for further use throughout the recommended treatment period. If, after full compression of the pump, the aerosol is not sprayed, or when the medicine has not been used for more than 6 days, the pump should be prepared again by pressing 4 times. Do not spray towards the eyes or mouth.
- 5. Hold the bottle upright, supporting the bottom of the bottle with your thumb, and place the tip between your two fingers.
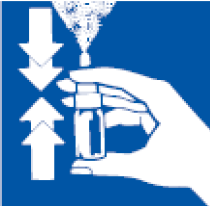
- 6. Tilt your head forward and insert the tip into the nostril.
- 7. Press the pump and inhale through the nose at the same time.

- 8. Repeat the steps when administering the medicine to the other nostril.
- 9. After use, clean and dry the tip of the dispenser and replace the protective cap.
For hygiene reasons, one package of the medicine should be used by only one patient.
Using a higher dose of Otrivin Menthol than recommended
In case of using a higher dose of the medicine than recommended, the patient should immediately consult a doctor or pharmacist.
Symptoms of overdose are severe dizziness, excessive sweating, significantly lowered body temperature, headache, slow heart rate, breathing difficulties, coma, convulsions, high blood pressure (hypertension), which may be followed by hypotension.
Symptomatic treatment should be applied.
Missing a dose of Otrivin Menthol
A double dose should not be used to make up for a missed dose.
If the patient has any further doubts about using this medicine, they should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Otrivin Menthol can cause side effects, although not everybody gets them.
The patient should STOP usingOtrivin Menthol and immediately consult a doctorif they experience any of the following side effects, which may be symptoms of an allergic reaction:
- difficulty breathing or swallowing,
- swelling of the face, lips, tongue, or throat,
- severe itching with red rash or blisters. These symptoms are very rare (less than 1 in 10,000 patients using the medicine).
Common (may affect up to 1 in 10 people using the medicine):
dryness or irritation of the nasal mucosa, discomfort in the nose, nausea, headache, burning sensation at the site of administration.
Uncommon (may affect up to 1 in 100 people using the medicine):
nosebleeds.
Rare (may affect up to 1 in 10,000 people using the medicine):
allergic reaction (skin rash, itching), transient vision disturbances, irregular or rapid heart rate.
Reporting side effects
If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Aleje Jerozolimskie 181C, 02-222 Warsaw, tel: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Otrivin Menthol
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Otrivin Menthol contains
- The active substance of Otrivin Menthol is xylometazoline hydrochloride. One ml of the solution contains 1 mg of xylometazoline hydrochloride.
- The other ingredients of the medicine are: sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, sodium chloride, disodium edetate, levomenthol, cineole (eucalyptol), sorbitol, macrogolglycerol hydroxystearate (hydrogenated polyoxyethylene castor oil), purified water.
What Otrivin Menthol looks like and contents of the pack
The packaging of Otrivin Menthol is a bottle with a dosing pump containing 10 ml of a white solution with a menthol and eucalyptol scent. The dosing pump allows for the administration of an exact dose of the medicine.
The packaging contains 60 doses.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Lithuania, the country of export:
Haleon Hungary Kft.
1124 Budapest, Csörsz utca 43
Hungary
Manufacturer:
Haleon Germany GmbH
Barthstraße 4
80339 Munich
Germany
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Lithuanian license number, in the country of export: LT/1/95/0102/002
Parallel import license number: 371/17
Date of leaflet approval: 26.05.2025
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Haleon Hungary Kft.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Otrivin MentholDosage form: Aerosol, 1mg/mlActive substance: xylometazolineManufacturer: Jadran-Galenski laboratorij d.d.Prescription not requiredDosage form: Aerosol, 0.5 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not requiredDosage form: Aerosol, 1 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not required
Alternatives to Otrivin Menthol in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Otrivin Menthol in Ucrania
Alternative to Otrivin Menthol in España
Online doctors for Otrivin Menthol
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Otrivin Menthol – subject to medical assessment and local rules.








