
Optilamid

Ask a doctor about a prescription for Optilamid

How to use Optilamid
Package Leaflet: Information for the Patient
Optilamid, 10 mg/ml, Eye Drops, Suspension
Brinzolamidum
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Optilamid and what is it used for
- 2. Important information before using Optilamid
- 3. How to use Optilamid
- 4. Possible side effects
- 5. How to store Optilamid
- 6. Contents of the pack and other information
1. What is Optilamid and what is it used for
Optilamid contains brinzolamide, which belongs to a group of medicines called carbonic anhydrase inhibitors. It lowers the pressure inside the eye.
Optilamid eye drops are used to treat high pressure in the eye. This pressure can lead to the development of a disease called glaucoma.
If the pressure in the patient's eye is too high, it can lead to vision loss.
2. Important information before using Optilamid
When not to use Optilamid
- if the patient has severe kidney function disorders
- if the patient is allergic to brinzolamide or any of the other ingredients of this medicine (listed in section 6)
- if the patient is allergic to sulphonamides. Examples of sulphonamides are medicines used to treat diabetes, infections, and diuretics; Optilamid may cause the same allergic reactions.
- if the patient has too much acid in the blood (a condition called hyperchloremic acidosis). In case of any further doubts, consult a doctor.
Warnings and precautions
Before starting to use Optilamid, discuss it with your doctor or pharmacist:
- if the patient has kidney or liver function disorders
- if the patient has dry eye or corneal disorders
- if the patient is taking other sulphonamide medicines
- -if the patient has ever had a severe skin rash or skin peeling, blistering, and (or) ulcers in the mouth after using brinzolamide or other related medicines.
Be careful when using brinzolamide:
Severe skin reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported with brinzolamide treatment. Stop using brinzolamide and seek medical attention immediately if you notice any symptoms related to these severe skin reactions described in section 4.
Children and adolescents
Optilamid should not be used in infants, children, and adolescents under 18 years of age, unless advised by a doctor.
Optilamid and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take, including those obtained without a prescription.
Tell your doctor about taking other carbonic anhydrase inhibitors (acetazolamide or dorzolamide, see section 1. What is Optilamid and what is it used for).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Women who can become pregnant are advised to use effective contraception during treatment with Optilamid. Optilamid should not be used during pregnancy or breastfeeding, unless the doctor decides otherwise.
Before using any medicine, consult a doctor or pharmacist.
Driving and using machines
Do not drive or operate machinery until your vision returns to normal. For some time after using Optilamid, your vision may be blurred.
Optilamid may affect your ability to perform tasks that require increased attention and (or) motor coordination. If such symptoms occur, be careful when driving or operating machinery.
Optilamid contains benzalkonium chloride
The medicine contains 0.15 mg of benzalkonium chloride per ml of suspension.
Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or corneal disorders (the transparent layer on the front of the eye). If you experience any abnormal sensations in the eye, stinging, or eye pain after using the medicine, contact your doctor.
Benzalkonium chloride may be absorbed by soft contact lenses and change their color. Remove contact lenses before using the drops and wait at least 15 minutes before putting them back.
3. How to use Optilamid
Always use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Optilamid is for use in the eye only. Do not swallow or inject it.
The recommended dose isone drop into the affected eye or eyes, twice a day - in the morning and evening.
Use the dose recommended by your doctor. Optilamid can be used in both eyes only if advised by a doctor. Use the medicine for as long as your doctor tells you.
Method of administration
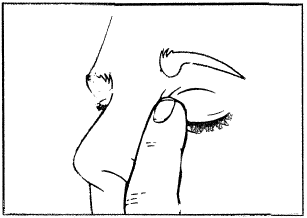
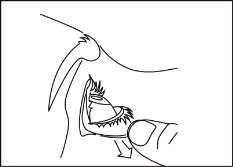
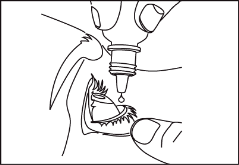
Figure 1. Figure 2. Figure 3.
- Prepare the Optilamid bottle and a mirror.
- Wash your hands.
- Shake the bottle and unscrew the cap. If the tamper-evident ring is loose after removing the cap, remove it before using the medicine.
- Hold the inverted bottle between your thumb and middle finger.
- Tilt your head back. With a clean finger, pull the lower eyelid down to create a "pocket"; the drop should fall into it (figure 1).
- Bring the tip of the bottle close to the eye. You can use a mirror to help.
- Do not touch the dropper to the eye or eyelid, or other surfaces. This may cause infection of the drops.
- Squeeze one drop of Optilamid into the formed "pocket" (figure 2).
- After using Optilamid, press the corner of your eye near your nose for at least 1 minute. This will help prevent the medicine from entering the entire body.
- If using drops in both eyes, repeat the above steps for the second eye.
- Immediately after use, screw the bottle cap back on.
- Open a new bottle only after the previous one is completely empty.
If you are using other eye drops, wait at least 5 minutes between using Optilamid and other eye drops. Apply eye ointments last.
Using more than the recommended dose of Optilamid
If you have accidentally used too many drops, rinse your eyes with lukewarm water. Do not use more drops until the next normal dose.
Missing a dose of Optilamid
If you miss a dose of Optilamid, use one drop in the eye as soon as you remember, and then return to your normal dosing schedule. Do not use a double dose to make up for a missed dose.
Stopping treatment with Optilamid
If you stop using Optilamid without consulting your doctor, the pressure in your eye will no longer be controlled, which can lead to vision loss.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed with brinzolamide.
Stop using brinzolamide and seek medical attention immediately if you notice any of the following symptoms:
- red, flat patches on the torso, often with blisters in the center, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin rashes may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis).
Common side effects(may affect up to 1 in 10 people)
Eyes:
- blurred vision, eye irritation, eye pain, eye discharge, eye itching, dry eye, abnormal sensations in the eye, redness of the eye.
General:
- unpleasant taste in the mouth.
Uncommon side effects(may affect up to 1 in 100 people)
Eyes:
- sensitivity to light, inflammation or infection of the conjunctiva, eye swelling, eyelid itching, redness or swelling of the eyelids, increased eye surface (conjunctivalization of the cornea), increased eye pigmentation, eye fatigue, eyelid crusts, increased tear production.
General:
- slow or weak heart rate, fast or irregular heart rate, breathing difficulties, shortness of breath, cough, reduced red blood cell count, increased chloride levels in the blood, dizziness, drowsiness, memory disorders, depression, nervousness, general weakness, fatigue, abnormal feeling, pain, chills, decreased libido, sexual dysfunction in men, cold symptoms, chest congestion, sinus infection, sore throat, abnormal sensation in the mouth, esophageal inflammation, stomach pain, nausea, vomiting, stomach upset, frequent bowel movements, diarrhea, gas in the intestines, digestive disorders, kidney pain, muscle pain, muscle cramps, back pain, nosebleeds, runny nose, nasal congestion, sneezing, rash, abnormal skin sensation, itching, headache, dry mouth.
Rare side effects(may affect up to 1 in 1,000 people)
Eyes:
- corneal edema, double or limited vision, abnormal vision, decreased sensation in the eye, swelling around the eye, increased eye pressure, optic nerve damage.
General:
- memory disorders, drowsiness, chest pain, upper respiratory tract congestion, sinus congestion, runny nose, dry nose, ringing in the ears, hair loss, generalized itching, feeling of shaking, irritability, irregular heart rate, weakness, difficulty sleeping.
Frequency not known(frequency cannot be estimated from the available data)
Eyes:
- eyelid abnormalities, vision disorders, corneal disease, eye allergy, reduced eyelash growth or number.
General:
- exacerbated allergic symptoms, decreased sensation, tremors, loss or decreased sense of taste, decreased blood pressure, increased blood pressure, increased heart rate, joint pain, asthma, limb pain, skin redness, skin inflammation or itching, abnormal liver function test results, limb swelling, frequent urination,
decreased appetite, red, flat patches on the torso, often with blisters in the center, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes, which may be preceded by fever and flu-like symptoms. These severe skin rashes can be life-threatening (Stevens-Johnson syndrome, toxic epidermal necrolysis).
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Optilamid
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after EXP. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
There are no special precautions for storage.
To prevent infections, the bottle should be discarded 4 weeks after first opening. The opening date of each bottle should be written in the space provided below and on the label of the bottle and carton. For packaging containing one bottle, enter only one date.
Opening date (1):
Opening date (2):
Opening date (3):
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Optilamid contains
- The active substance is brinzolamide. Each ml of suspension contains 10 mg of brinzolamide. Each drop contains 0.27 mg of brinzolamide.
- The other ingredients are: benzalkonium chloride, mannitol (E421), carbomer 974P, disodium edetate, sodium chloride, purified water. Additionally, small amounts of hydrochloric acid or sodium hydroxide are added to maintain the acidity (pH) of the medicine.
What Optilamid looks like and contents of the pack
Optilamid is a white or almost white homogeneous suspension.
Optilamid is available in a sterile polyethylene (LDPE) bottle of 10 ml containing 5 ml of suspension, with a polyethylene dropper (LDPE) and a HDPE cap with a tamper-evident seal.
Pack sizes: 1 or 3 bottles.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A.
Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
Pelplińska 19, 83-200 Starogard Gdański
Lusomedicamenta Sociedade Técnica Farmacêutica, S.A.
Rua Norberto de Oliveira, no 1/5, Póvoa de Santo Adrião, 2620-111,
Portugal
Date of last revision of the leaflet:January 2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLusomedicamenta Sociedade Tecnica Farmaceutica, S.A. Zakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OptilamidDosage form: Drops, 10 mg/mlActive substance: brinzolamideManufacturer: Lusomedicamenta Sociedade Tecnica Farmaceutica, S.A.Prescription not requiredDosage form: Drops, 10 mg/mlActive substance: brinzolamideManufacturer: Balkanpharma-Razgrad AD Famar S.A., Plant A Heumann Pharma GmbH & Co. Generica KG Pharmaten S.A.Prescription requiredDosage form: Tablets, 250 mgActive substance: acetazolamide
Alternatives to Optilamid in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Optilamid in Ukraine
Alternative to Optilamid in Spain
Online doctors for Optilamid
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Optilamid – subject to medical assessment and local rules.














