
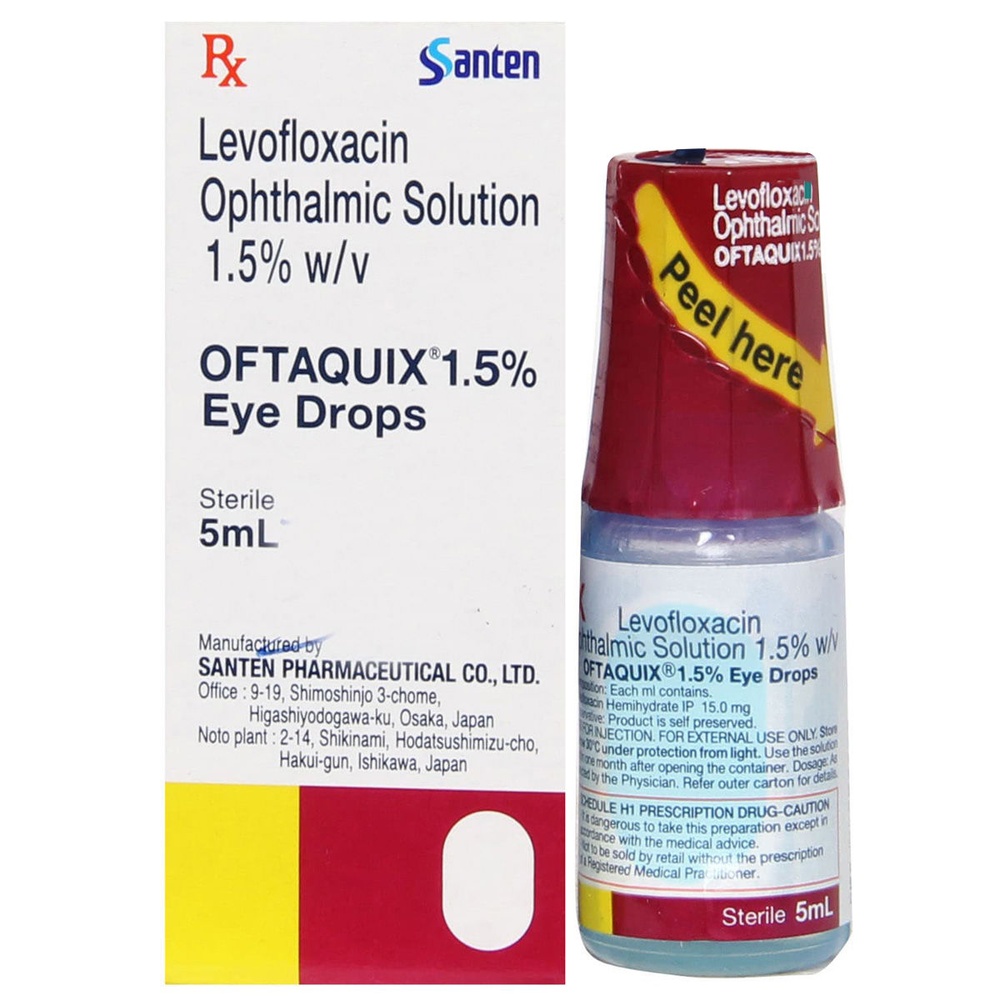
Oftaquix

Ask a doctor about a prescription for Oftaquix

How to use Oftaquix
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
OFTAQUIX
5 mg/ml, eye drops, solution
Levofloxacin
Table of contents of the leaflet:
- 1. What is Oftaquix and what is it used for
- 2. Important information before using Oftaquix
- 3. How to use Oftaquix
- 4. Possible side effects
- 5. How to store Oftaquix
- 6. Contents of the pack and other information
1. What is Oftaquix and what is it used for
The active substance of Oftaquix is levofloxacin. Levofloxacin is a synthetic fluoroquinolone antibiotic. It has a bactericidal effect on some types of bacteria that can cause infections.
Levofloxacin in the form of eye drops is used in children over 1 year of age and adults for the treatment of bacterial infections affecting the front surface of the eye.
2. Important information before using Oftaquix
When not to use Oftaquix:
- If the patient has been found to be hypersensitive (allergic) to levofloxacin or to any other fluoroquinolone antibiotic, or to any of the other ingredients of the medicine.
- If the patient is less than 1 year old.
- If the patient is pregnant or breastfeeding.
When to exercise special caution when using Oftaquix
If during treatment the patient observes an increase in eye symptoms or if after a certain period of treatment established by the doctor no signs of recovery are visible, they should contact their doctor immediately.
Oftaquix should not be injected subconjunctivally or administered directly into the anterior chamber of the eye.
During general administration of fluoroquinolone drugs, hypersensitivity reactions have been observed, even after a single dose. In the event of an allergic reaction to
You should read the contents of the leaflet before using the medicine.
- You should keep this leaflet, so that you can read it again if you need to.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
levofloxacin, you should stop using Oftaquix and contact your doctor as soon as possible.
Swelling and tendon rupture have occurred in people taking fluoroquinolones orally or intravenously, especially in older patients and those treated with corticosteroids at the same time. You should stop treatment with Oftaquix if you experience pain or swelling of the tendon (tendinitis).
Using Oftaquix with other medicines
You should tell your doctor about all the medicines you have taken recently, including those that are available without a prescription. In particular, you should tell your doctor or pharmacist about using any other eye drops or ointments before starting treatment with Oftaquix.
If you are using other eye drops, you should wait at least 15 minutes between administering Oftaquix and other eye drops.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, you should consult your doctor or pharmacist before taking any medicine.
Using Oftaquix in pregnant or breastfeeding women is contraindicated.
Driving and operating machinery
If the eye drops cause blurred vision, you should wait until your vision is clear before driving or operating machinery.
Oftaquix contains benzalkonium chloride
This medicine contains about 0.002 mg of benzalkonium chloride per drop, which corresponds to 0.05 mg/ml. Benzalkonium chloride may be absorbed by soft contact lenses and change their color. You should remove your contact lenses before administering the drops and wait at least 15 minutes before putting them back in.
Benzalkonium chloride can also cause eye irritation, especially in people with dry eye syndrome or disorders affecting the cornea (the transparent layer at the front of the eye). If you experience any abnormal sensations in the eye, stinging, or pain in the eye after using the medicine, you should contact your doctor.
3. How to use Oftaquix
Oftaquix should always be used in accordance with the doctor's recommendations. If you have any doubts, you should contact your doctor again.
Usually, one or two drops are administered into the conjunctival sac of the infected eye or eyes every two hours (up to eight times a day) except during sleep, for the first two days, and then four times a day for the next three days.
Usually, treatment lasts for five days, but sometimes longer treatment cycles are used. Your doctor will inform you how long you should use the drops.
If you are using any other eye medicine, you should wait at least 15 minutes between administering different types of eye drops.
Before administering the drops:
- 1) Wash your hands.
- 2) Open the bottle. Be careful not to touch the dropper tip to the eyelid or surrounding areas or any other surface. If this happens accidentally, you should tell your doctor or pharmacist so that you can get a clean bottle.Tilt your head back in a sitting or lying position.

- 3) Bring the bottle tip close to the eye without touching it.

- 4) Pull the lower eyelid down and look up.

- 5) Squeeze the bottle gently to allow one drop to fall into the space between the lower eyelid and the eye.

After administering the medicine, it is recommended to press the inner corner of the eye with your index finger or close your eyes for 3 minutes. This prevents the medicine from passing through the tear duct into the nose or throat and increases the amount of medicine that stays in the eye and acts locally.
- 6) If an additional drop is needed, and if the treatment involves both eyes, repeat the actions described in steps 3 to 6. After use, carefully close the dropper cap again.
Using more Oftaquix than recommended:rinse the eye (eyes) with clean water and inform your doctor or pharmacist.
Missing a dose of Oftaquix:use the next dose as soon as you remember. Do not use a double dose to make up for a missed dose.
Accidental ingestion of eye drops
The amount of levofloxacin contained in the bottle is too small to cause side effects. However, in case of doubt, you should inform your doctor or pharmacist, who will recommend the necessary measures.
4. Possible side effects
Like all medicines, Oftaquix can cause side effects, although not everybody gets them. If you experience any serious or persistent side effects, you should stop using Oftaquix and seek urgent medical attention.
There is a possibility of an allergic reaction to Oftaquix after just one dose. In such a situation, redness or itching of the eyes or swelling of the eyelids may occur. If this happens, you should stop using Oftaquix and contact your doctor immediately.
Side effects may occur with the following frequency:
Very common: in more than 1 in 10 patients
Common: in less than 1 in 10 but more than 1 in 100 patients
Uncommon: in less than 1 in 100 but more than 1 in 1,000 patients
Rare: in less than 1 in 1,000 but more than 1 in 10,000 patients
Very rare: in less than 1 in 10,000 patients.
Common: eye burning sensation, blurred vision, or mucous discharge in the conjunctival sac.
Uncommon: eyelid discoloration, conjunctival edema, conjunctival papillary reaction, eyelid swelling, eye discomfort, eye itching, eye pain, conjunctival hyperemia, conjunctival nodular reaction, eye dryness, eyelid erythema and photophobia, eyelid burning sensation, dry residue on the eyelid margins, tearing, headache, rhinitis.
Rare: non-ocular allergic reactions, including skin rash.
Very rare: laryngeal edema, severe allergic reactions, such as sudden shortness of breath and chest tightness, eyelid swelling, face or mouth swelling.
Additionally, nausea may occur.
If you experience any side effects with a sudden onset or rapid worsening, you should stop treatment and contact your doctor or hospital emergency department immediately.
If you experience any other side effects not mentioned in this leaflet, you should inform your doctor or pharmacist.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of the medicine.
5. How to store Oftaquix
Oftaquix should be stored out of sight and reach of children.
Store the bottle tightly closed.
Do not use this medicine after the expiry date. The expiry date is the last day of the month stated.
Oftaquix retains its potency for 28 days after the first opening of the bottle.
Unused medicine should be returned to the pharmacy for safe disposal.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Oftaquix contains
The active substance is levofloxacin 5 mg/ml in the form of 5.12 mg/ml levofloxacin hemihydrate. One bottle of Oftaquix contains 5 ml of solution.
The other ingredients are: benzalkonium chloride, sodium chloride, sodium hydroxide or hydrochloric acid, water for injections.
What Oftaquix looks like and contents of the pack
Eye drops, solution in a 5 ml bottle in a cardboard box.
For more detailed information, you should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Portugal, the country of export:
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer:
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Authorization number in Portugal, the country of export:4041786
Parallel import authorization number:307/17
Date of leaflet approval: 08.07.2022
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Santen Oy
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OftaquixDosage form: Drops, 5 mg/mlActive substance: levofloxacinManufacturer: Rafarm S.A.Prescription not requiredDosage form: Drops, 5 mg/mlActive substance: levofloxacinPrescription requiredDosage form: Drops, 5 mg/mlActive substance: levofloxacinManufacturer: Unimed Pharma spol. s.r.o.Prescription required
Alternatives to Oftaquix in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Oftaquix in Ukraine
Alternative to Oftaquix in Spain
Online doctors for Oftaquix
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Oftaquix – subject to medical assessment and local rules.














