
Niscandin

Ask a doctor about a prescription for Niscandin

How to use Niscandin
Leaflet attached to the packaging: patient information
Nyscandin, 100 000 IU/mL oral suspension
Nystatin
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Nyscandin and what is it used for
- 2. Important information before taking Nyscandin
- 3. How to take Nyscandin
- 4. Possible side effects
- 5. How to store Nyscandin
- 6. Contents of the packaging and other information
1. What is Nyscandin and what is it used for
Nystatin is a polyene antibiotic with antifungal activity - fungistatic or fungicidal (due to damage to the fungal cell membrane). Nystatin acts on many strains of yeast and yeast-like fungi, particularly on Candida species (including Candida albicans).
The medicine is not absorbed into the body fluids and only acts locally at the site of administration.
Indications:
- treatment of yeast infections of the gastrointestinal tract, including the mouth (e.g., oral thrush) and esophagus;
- prophylactically in patients treated with high doses of antibiotics or corticosteroids;
- prophylactically in newborns with a birth weight below 1500 g, in whom the first-choice medicine cannot be used.
2. Important information before taking Nyscandin
When not to take Nyscandin
- if the patient is allergic to nystatin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to take Nyscandin, discuss it with your doctor or pharmacist.
Do not use oral forms of nystatin to treat systemic fungal infections (infections of internal organs or skin).
In patients with renal failure, nystatin may exceptionally appear in small concentrations in the blood.
Nyscandin and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
There is no data on the interaction of nystatin with other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before taking this medicine.
It is not known whether the medicine can harm the fetus.
Nyscandin is not recommended during pregnancy.
The medicine should not be used during breastfeeding.
Driving and using machines
The effect of the medicine on the ability to drive and use machines is not known.
Nyscandin contains sucrose, methyl parahydroxybenzoate, propyl parahydroxybenzoate, propylene glycol, and sodium
Sucrose
The medicine contains 489.22 mg of sucrose in each 1 mL of suspension.
This should be taken into account in patients with diabetes.
If you have previously been diagnosed with intolerance to some sugars, consult your doctor before taking the medicine.
Methyl and propyl parahydroxybenzoate
The medicine may cause allergic reactions (possible late reactions).
Propylene glycol
The medicine contains 5.62 mg of propylene glycol in each 1 mL of suspension. Concurrent administration with other substrates of alcohol dehydrogenase, such as ethanol, may cause severe side effects in newborns.
Sodium
The medicine contains less than 1 mmol (23 mg) of sodium in 1 mL of suspension, which means the medicine is considered "sodium-free".
3. How to take Nyscandin
Always take this medicine exactly as your doctor has told you. If you are not sure, consult your doctor or pharmacist.
1 mL of suspension contains 100 000 IU of nystatin.
Adults
Treatment of yeast infections of the mouth and throat:
400 000 to 600 000 IU, which corresponds to 4 mL to 6 mL of suspension, 4 times a day, every 6 hours.
The suspension should be kept in the mouth for as long as possible before swallowing.
Treatment of yeast infections of the gastrointestinal tract:
500 000 IU to 1 000 000 IU, which corresponds to 5 mL to 10 mL of suspension, 4 times a day, every 6 hours.
Prophylactically (during antibiotic therapy):
500 000 IU, which corresponds to 5 mL of suspension, 3 times a day, every 8 hours.
Use in infants, children, and adolescents
Treatment of yeast infections of the mouth:
Oral thrush: 100 000 IU, which corresponds to 1 mL of suspension, 4 times a day, every 6 hours.
The longer the suspension stays in the mouth in contact with the infected area, the more effective the medicine will be.
Treatment of yeast infections of the gastrointestinal tract:
50 000 IU to 500 000 IU, which corresponds to 0.5 mL to 5 mL of suspension, 4 times a day, every 6 hours.
Prophylaxis of yeast infections in newborns with a birth weight below 1500 g:
100 000 IU, which corresponds to 1 mL of suspension, 3 times a day, every 8 hours, for 6 weeks.
The medicine can also be used externally to brush the mouth 2 to 3 times a day. The product should be applied to the affected areas using a hygiene swab moistened with the suspension.
Method of administration
Oral administration.
Before each use, shake the bottle thoroughly.A measuring spoon with an adapter is provided with the packaging to facilitate dosing.
Instructions for using the measuring spoon with an adapter are provided at the end of this leaflet.
Using more than the recommended dose of Nyscandin
Nystatin is practically not absorbed from the gastrointestinal tract, and overdose or accidental ingestion does not cause toxic systemic effects.
If you have taken more than the recommended dose of the medicine, consult your doctor or pharmacist immediately.
Missing a dose of Nyscandin
Take the missed dose as soon as possible. If it is almost time for the next dose, take it at the scheduled time. Do not take a double dose to make up for the missed dose.
If you have any further doubts about taking this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Nyscandin can cause side effects, although not everybody gets them.
Nystatin is well-tolerated, even during long-term use.
Side effects are listed below by frequency of occurrence.
Rare(occurring in 1 to 10 people out of 10,000):
- nausea;
- vomiting;
- diarrhea;
- allergic reactions (e.g., rash, urticaria).
Very rare(occurring in less than 1 person out of 10,000):
- Stevens-Johnson syndrome - occurrence of blisters on the skin and/or mucous membranes, which form painful sores after rupture, often accompanied by fever, muscle and joint pain.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, inform your doctor or pharmacist.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Nyscandin
Keep the medicine out of sight and reach of children.
Store in a temperature below 30°C. Do not store in the refrigerator or freeze.
Do not use this medicine after the expiry date stated on the carton and label. The expiry date refers to the last day of the month.
Shelf life after first opening the bottle: 3 months.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Nyscandin contains
- The active substance is nystatin. 1 mL of suspension contains 100 000 IU of nystatin.
- The other ingredients are: sucrose, methyl parahydroxybenzoate (E 218), propyl parahydroxybenzoate (E 216), glycerol (E 422), xanthan gum, hydroxypropyl cellulose, citric acid (to adjust pH 6.0-7.0), sodium citrate (to adjust pH 6.0-7.0), raspberry flavor AR0320 (Himbeere fl. (mixture of flavoring substances and solvent), cis-3-hexenol, vanillin, propylene glycol), purified water.
What Nyscandin looks like and what the packaging contains
The medicine is a yellow oral suspension with a sweet-bitter taste and a raspberry smell.
The packaging is a brown glass bottle (type III) with a child-resistant closure and a 5 mL oral syringe with a 0.1 mL graduation and an adapter, in a cardboard box.
The packaging contains 30 mL or 70 mL of suspension.
Marketing authorization holder
Aflofarm Farmacja Polska Sp. z o.o.
ul. Partyzancka 133/151
95-200 Pabianice
phone: (42) 22-53-100
Manufacturer
Aflofarm Farmacja Polska Sp. z o.o.
ul. Krzywa 2
95-030 Rzgów
Date of last revision of the leaflet:
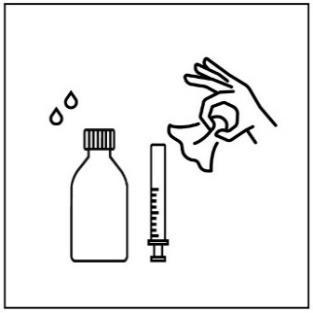
Instructions for using the measuring spoon with an adapter
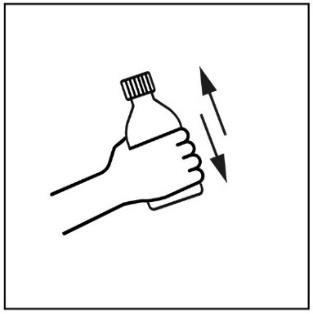
- 1. Before each use, shake the bottle thoroughly
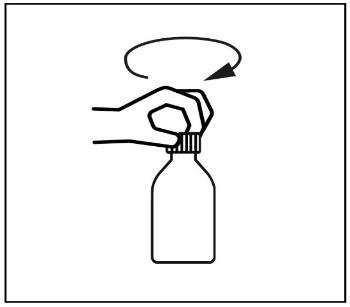
- 2. Unscrew the bottle cap
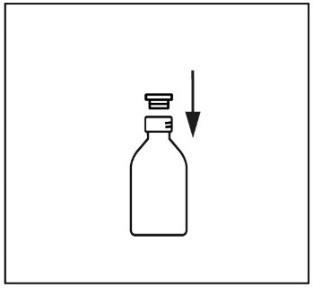
- 3. Place the provided adapter into the bottle neck
- 4. Push the syringe firmly into the adapter recess
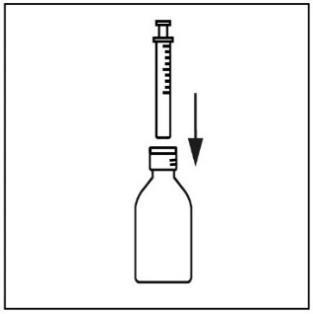
- 5. To fill the syringe, turn the bottle upside down and carefully push the syringe plunger down, drawing the suspension to the desired mark on the graduation
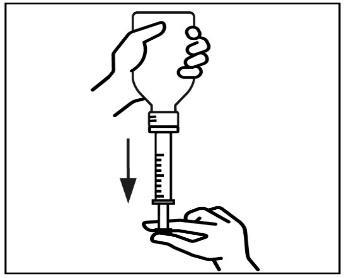
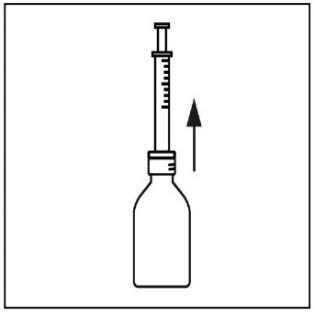
- 6. Turn the bottle back to its original position and carefully remove the syringe from the bottle
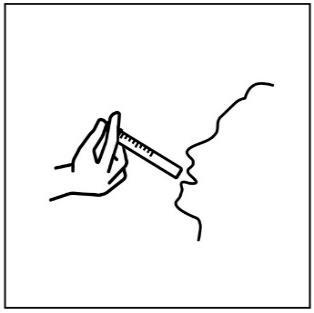
- 7. Place the syringe tip in your mouth and, pressing the plunger slowly, carefully administer the suspension to avoid choking
- 8. After use, close the bottle and wash and dry the syringe
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterAflofarm Farmacja Polska Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NiscandinDosage form: Powder, 100000 IU/mlActive substance: nystatinManufacturer: Tarchomińskie Zakłady Farmaceutyczne "Polfa" S.A.Prescription requiredDosage form: Tablets, 500,000 IUActive substance: nystatinManufacturer: Teva Operations Polska Sp. z o.o.Prescription required
Alternatives to Niscandin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Niscandin in Ukraine
Alternative to Niscandin in Spain
Online doctors for Niscandin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Niscandin – subject to medical assessment and local rules.














