
HUMATIN 250 mg HARD CAPSULES

How to use HUMATIN 250 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet:information for the patient
Humatin 250 mg hard capsules
paromomycin
Read this leaflet carefully before starting to take this medicine,as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What Humatin 250 mg hard capsules are and what they are used for
- What you need to know before taking Humatin 250 mg hard capsules
- How to take Humatin 250 mg hard capsules
- Possible side effects
5 Storage of Humatin 250 mg hard capsules
- Contents of the pack and further information
1. What Humatin 250 mg hard capsules are and what they are used for
Humatin 250 mg hard capsules contain paromomycin, which belongs to a group of antibiotics called aminoglycoside antibiotics. It is used to treat intestinal infections and hepatic coma.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or common cold.
It is essential to follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medicine. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not throw medicines down the drain or in the trash.
Humatin 250 mg hard capsules (paromomycin) are indicated in children and adults for the treatment of:
- Acute and chronic non-invasive intestinal amebiasis.
In addition, it is indicated in adults for:
- Adjunctive treatment of hepatic coma by reducing ammonia-forming bacteria in the gastrointestinal tract.
2. What you need to know before taking Humatin 250 mg hard capsules
Do not take Humatin250 mghard capsules
- if you are allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
- if you have been previously treated with paromomycin and have developed hypersensitivity reactions
- if you have had an allergic reaction to any other aminoglycoside antibiotic, as you may also be allergic to paromomycin
- if you have pre-existing ear damage (dizziness, vertigo, ringing in the ears, or hearing loss)
- if you have pathological muscle weakness (myasthenia gravis)
- if you have constipation or intestinal obstruction
- during pregnancy and breastfeeding
Due to the possible risk of damage to renal and auditory function, the active ingredient paromomycin contained in Humatin 250 mg capsules should not be administered without passing through the gastrointestinal tract (parenterally).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to take Humatin 250 mg hard capsules if:
- you have renal insufficiency
- you are undergoing long-term treatment (e.g., treatment of hepatic coma by reducing ammonia-forming bacteria in the gastrointestinal tract)
- you have gastrointestinal ulcers or bleeding in the intestines
In the above-mentioned patient groups, auditory function should be regularly monitored.
If signs of ototoxicity (dizziness, vertigo, ringing in the ears, or hearing loss) or nephrotoxicity appear, it may be necessary to reduce the dose or even consider discontinuing treatment.
Prolonged and/or repeated use of Humatin 250 mg capsules may cause a new or secondary infection by bacteria or fungi insensitive to paromomycin (resistant). Be aware of the signs of a possible subsequent infection with these pathogens (fungal infection of the mucous membranes with redness and white coatings on the mucous membranes).
These infections should be treated accordingly.
If you experience intense, persistent, sometimes bloody mucous diarrhea and abdominal pain similar to cramps during or after treatment with Humatin 250 mg capsules, you should inform your doctor because this is due to a severe inflammation of the mucous membranes of the intestine (pseudomembranous enterocolitis) usually caused by Clostridioides difficileand should be treated immediately. This intestinal disease caused by antibiotic treatment can be life-threatening.
Other medicines and Humatin250 mghard capsules
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
When taking paromomycin, only a very small amount is absorbed into the bloodstream through the gastrointestinal tract, so interactions with other medicines in the body are unlikely. In theory, the same interactions that occur with systemically administered aminoglycosides may occur.
With the simultaneous use of certain muscle relaxants (non-depolarizing muscle relaxants), the blockade of stimulus transmission between nerve and muscle may be deepened and prolonged.
Due to the increased risk of side effects, special monitoring is required for patients who are being treated simultaneously or subsequently with other medicines that may be harmful to renal or auditory function, such as amphotericin B, colistin, cyclosporine, cisplatin, vancomycin, or certain diuretic drugs such as ethacrynic acid and furosemide.
Pregnancy, breastfeedingand fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Humatin 250 mg hard capsules should not be used during pregnancy and breastfeeding.
Driving and using machines
No data are available on the influence of paromomycin on the ability to drive and use machines.
3. How to take Humatin 250 mg hard capsules
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Your doctor will determine the most suitable dose and treatment duration for you, according to your condition and response to treatment. It is administered orally. As a general rule, the recommended dose of the medicine and the frequency of administration are as follows:
Non-invasive intestinal amebiasis:
Adults, adolescents, and children who can swallow capsules: the recommended total daily dose is 25-35 mg/kg/day, divided into 3 doses, administered with meals, for 5 days.
Hepatic coma:
Adults: 4 g/day, divided into 2-4 doses, for 5-6 days.
Paromomycin sulfate capsules should only be used in adults, adolescents, and children who can swallow capsules.
The capsule should be taken whole.
Special patient groups
Patient with hepatic insufficiency
Generally, no dose adjustment is necessary in patients with hepatic insufficiency.
Patient with renal insufficiency
Paromomycin that is absorbed into the bloodstream is mainly eliminated through the kidneys. Therefore, paromomycin should be used with caution in patients with renal insufficiency.
If you take more Humatin250 mghard capsules than you should
Since the active ingredient of Humatin 250 mg capsules, paromomycin, is barely absorbed into the bloodstream when the mucous membrane of the gastrointestinal tract is intact, it is unlikely that symptoms of overdose will occur with a small overdose of Humatin 250 mg capsules (see, however, regarding the possibility of ototoxicity in section 2).
If you suspect an overdose, inform your doctor so that they can decide how to proceed.
If you forget to take Humatin 250 mg hard capsules
Do not take a double dose to make up for forgotten doses.
In this case, continue taking the medicine at the next scheduled time according to the usual dosing schedule.
If you do not notice your error until the next day, do not take a double dose to make up for forgotten doses, and continue taking the same amount of Humatin 250 mg capsules as prescribed. It may be necessary to extend the treatment by one day. In this case, consult your doctor. You should also consult your doctor if you forget to take a dose or take too little.
If you stop taking Humatin 250 mg hard capsules
Treatment with Humatin 250 mg hard capsules should never be stopped on your own initiative, and you should not interrupt it without medical advice. You should also not change the prescribed dose on your own. It is imperative that you strictly adhere to the dose prescribed by your doctor. The success of treatment with Humatin 250 mg hard capsules depends on completing the entire treatment.
If you have any further questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you think any of the side effects you are experiencing is serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Common (may affect up to 1 in 10 people):
- soft stools,
- diarrhea
Rare (may affect up to 1 in 1,000 people):
- anorexia,
- nausea,
- vomiting,
- gastric cramps,
- abdominal or stomach pain,
- digestive disorders with fatty stools (malabsorption syndrome)
- allergic reactions such as hives
Very rare (may affect up to 1 in 10,000 people):
- pancreatitis (inflammation of the pancreas),
- headache,
- dizziness,
- increase in the number of certain white blood cells in the blood (eosinophilia)
- blood in the urine (hematuria)
Reporting of side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Agency's website: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Humatin 250 mg hard capsules
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP. The expiry date is the last day of the month indicated.
Store at room temperature.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicine to the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Humatin250 mghard capsules
- The active substance is paromomycin. Each hard capsule contains 250 mg of paromomycin.
- The other ingredients (excipients) are:
Capsule content: magnesium stearate, anhydrous colloidal silica
Capsule composition: gelatin, titanium dioxide (CI = 77891) (E-171), yellow iron oxide (CI = 77492) (E 172), red iron oxide (CI = 77491) (E 172), black iron oxide (CI = 77499) (E 172), 1-butanol, Shellac gum, Shellac lacquer, isopropyl alcohol, ammonia, propylene glycol, antifoam C.
Appearance of the product and contents of the pack
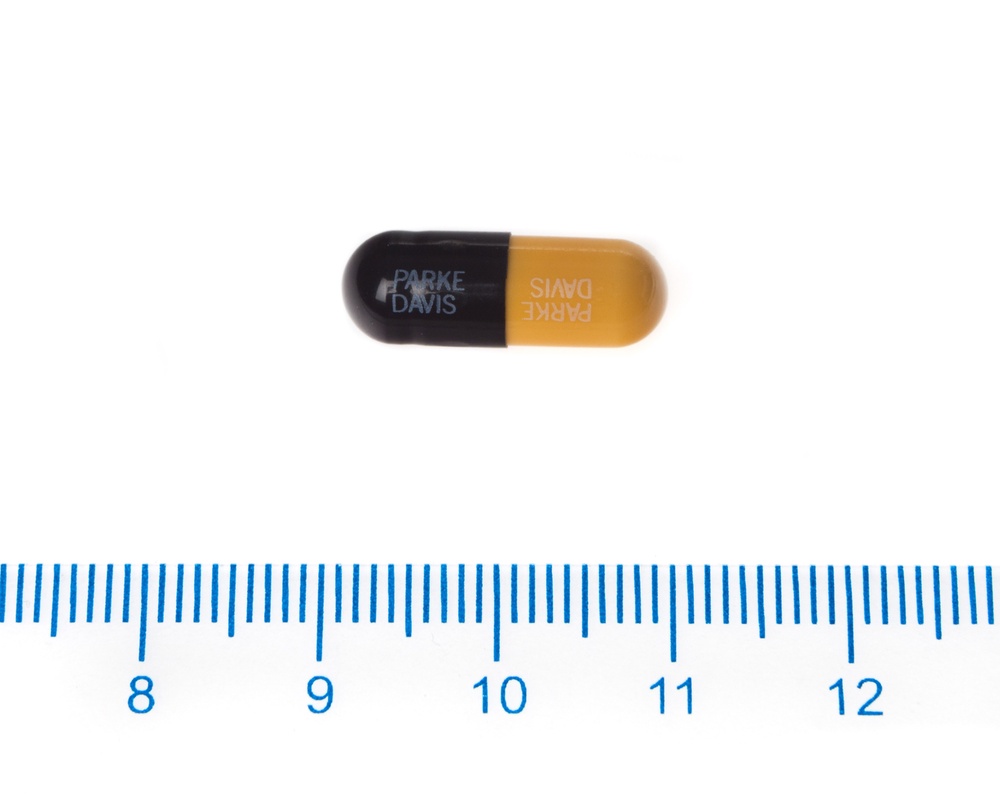
Humatin 250 mg hard capsules are presented in the form of hard capsules with a yellow body and a black cap. The pack contains 8 capsules.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Pfizer, S.L.
Avda. de Europa, 20 B.
Parque Empresarial La Moraleja.
28108 Alcobendas. Madrid
Spain.
Manufacturer:
Delpharm L'aigle.
Zone Industrielle Nº 1, Route de Crulai - L'Aigle – 61300
France
Date of the last revision of this leaflet: February 2022
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/)
-
- Country of registration
- Average pharmacy price6.24 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HUMATIN 250 mg HARD CAPSULESDosage form: TABLET, 200 mg fidaxomicinActive substance: fidaxomicinManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 40 mg/mlActive substance: fidaxomicinManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 100,000 IU nystatin/mlActive substance: nystatinManufacturer: SubstipharmPrescription required
Online doctors for HUMATIN 250 mg HARD CAPSULES
Discuss questions about HUMATIN 250 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















