
Neoparin

Ask a doctor about a prescription for Neoparin

How to use Neoparin
Leaflet attached to the packaging: patient information
Neoparin, 2000 IU (20 mg)/0.2 ml, solution for injection
Neoparin, 4000 IU (40 mg)/0.4 ml, solution for injection
Neoparin, 6000 IU (60 mg)/0.6 ml, solution for injection
Neoparin, 8000 IU (80 mg)/0.8 ml, solution for injection
Neoparin, 10,000 IU (100 mg)/1 ml, solution for injection
Enoxaparin sodium
You should carefully read the contents of this leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, you should tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Neoparin and what is it used for
- 2. Important information before using Neoparin
- 3. How to use Neoparin
- 4. Possible side effects
- 5. How to store Neoparin
- 6. Contents of the packaging and other information
1. What is Neoparin and what is it used for
Neoparin contains the active substance enoxaparin sodium, which is a low molecular weight heparin (LMWH).
Neoparin works in two ways.
- 1) It prevents the growth of existing blood clots. This helps the body to dissolve existing blood clots, making them no longer harmful.
- 2) It prevents the formation of new blood clots in the patient's blood.
Neoparin can be used for:
- Treating blood clots that are already present in the patient's blood.
- Preventing the formation of blood clots in the patient's blood in the following situations:
- Before and after surgery
- During an acute illness, when the patient has limited mobility
- In patients who have developed blood clots in the circulating blood due to cancer, to further prevent the formation of new blood clots
- In unstable angina (a condition in which the heart does not receive enough blood)
- After a heart attack
- Preventing the formation of clots in dialysis tubes (used in people with severe kidney function disorders).
2. Important information before using Neoparin
When not to use Neoparin
- If the patient is allergic to enoxaparin sodium or any of the other ingredients of this medicine (listed in section 6). Symptoms of an allergic reaction may include: rash, difficulty swallowing or breathing, swelling of the lips, face, throat, or tongue.
- If the patient has been diagnosed with an allergy to heparin or other low molecular weight heparins, such as nadroparin, tinzaparin, or dalteparin.
- If the patient has been diagnosed with a reaction to heparin that caused a significant decrease in the number of blood cells responsible for blood clotting (platelets) - a reaction known as heparin-induced thrombocytopenia - within the last 100 days or if there are antibodies against enoxaparin in the patient's blood.
- If the patient has severe bleeding or a medical condition associated with an increased risk of bleeding (e.g., stomach ulcers, recent brain or eye surgery), including recent hemorrhagic stroke.
- If the patient is using Neoparin to treat blood clots and spinal or epidural anesthesia or lumbar puncture is planned within 24 hours.
Warnings and precautions
Neoparin should not be used interchangeably with medicines containing other low molecular weight heparins.
This is because they are not exactly the same, differ in activity and administration instructions.
Before starting to use Neoparin, you should discuss it with your doctor or pharmacist if:
- the patient has ever had a reaction to heparin that caused a significant decrease in the number of platelets
- the patient is planned to have spinal or epidural anesthesia or lumbar puncture (see "Surgical procedures and anesthetics"): an appropriate time interval should be considered between the use of Neoparin and this procedure
- the patient has a heart valve replacement
- the patient has endocarditis (infection of the membrane lining the heart from the inside)
- the patient has had stomach ulcers
- the patient has recently had a brain stroke
- the patient has high blood pressure
- the patient has diabetes or has problems with blood vessels in the eyes caused by diabetes (so-called diabetic retinopathy)
- the patient has recently had eye or brain surgery
- the patient is elderly (over 65 years), especially if they are over 75 years old
- the patient has kidney disease
- the patient has liver disease
- the patient is underweight or overweight
- the patient has an increased level of potassium in the blood (this can be checked in a blood test)
- the patient is currently taking medicines that may cause bleeding (see the section below "Neoparin and other medicines")
Before starting to use this medicine and periodically during its use, the patient may have a blood test; it is intended to check the number of platelets responsible for blood clotting and potassium in the patient's blood.
Neoparin and other medicines
You should tell your doctor or pharmacist about all medicines you are taking now or have taken recently, as well as any medicines you plan to take.
- Warfarin - a medicine used to thin the blood
- Aspirin (also known as acetylsalicylic acid or ASA), clopidogrel, or other medicines used to prevent blood clots (see also section 3 "Change of anticoagulant medicine")
- Dextrans injections - used as a blood substitute
- Ibuprofen, diclofenac, ketorolac, or other non-steroidal anti-inflammatory medicines used to treat pain and swelling in arthritis and other conditions
- Prednisolone, dexamethasone, or other medicines used to treat asthma, rheumatoid arthritis, and other conditions
- Medicines that increase potassium levels in the blood, such as potassium salts, diuretics, and certain heart medicines.
Surgical procedures and anesthetics
If the patient has a planned lumbar puncture or surgical procedure under spinal or epidural anesthesia, they should inform their doctor that they are using Neoparin. See the section "When not to use Neoparin". Additionally, the patient should inform their doctor if they have any spinal problems or if they have had spinal surgery.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
In pregnant women with a mechanical heart valve, there may be an increased risk of blood clots. The doctor should discuss this with the patient.
Women who are breastfeeding or plan to breastfeed should consult their doctor before starting to use this medicine.
Driving and using machines
Neoparin does not affect the ability to drive or use machines.
The doctor should document the trade name and batch number of the product used.
3. How to use Neoparin
This medicine should always be used exactly as directed by your doctor or pharmacist. If you are unsure, you should consult your doctor or pharmacist.
Administration of the medicine
- Neoparin will usually be administered to the patient by a doctor or nurse. This is because it requires injection.
- After returning home, the patient may need to continue using Neoparin and administer it themselves (see the administration instructions below).
- Neoparin is usually administered by subcutaneous injection.
- Neoparin may be administered intravenously in certain types of heart attack or after surgery.
- Neoparin may be introduced into the dialysis tube returning blood from the body (into the so-called arterial line) at the beginning of the dialysis session.
- Neoparin must not be administered intramuscularly.
Dose of the medicine
- The doctor will decide what dose of Neoparin the patient should take. This depends on the reason for using the medicine.
- In patients with kidney disease, the patient may receive a lower dose of Neoparin.
- 1. Treatment of blood clots in the patient's blood
- The usual dose is 150 IU (1.5 mg) per kilogram of body weight once daily or 100 IU (1 mg) per kilogram of body weight twice daily.
- The doctor will decide how long the patient should receive Neoparin.
- 2. Prevention of blood clot formation in the patient's blood in the following situations:
- Surgical procedure or period of limited mobility due to illness
- The dose depends on the patient's risk of blood clot formation. The patient will receive Neoparin at a dose of 2000 IU (20 mg) or 4000 IU (40 mg) daily.
- In the case of planned surgery, the first injection is usually performed 2 hours or 12 hours before the procedure.
- If the patient has limited mobility due to illness, they will usually receive Neoparin at a dose of 4000 IU (40 mg) daily.
- The doctor will decide how long the patient should receive Neoparin.
- After a heart attackNeoparin can be used in two different types of heart attack: ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI). The dose of Neoparin will depend on the patient's age and the type of heart attack they had.
Non-ST-segment elevation myocardial infarction (NSTEMI):
- The usual dose is 100 IU (1 mg) per kilogram of body weight every 12 hours.
- The doctor will usually recommend that the patient also take aspirin (acetylsalicylic acid).
- The doctor will decide how long the patient should receive Neoparin.
ST-segment elevation myocardial infarction (STEMI) in patients under 75 years of age:
- The initial dose of Neoparin is 3000 IU (30 mg) administered intravenously.
- Neoparin will also be administered subcutaneously. The usual dose is 100 IU (1 mg) per kilogram of body weight every 12 hours.
- The doctor will usually recommend that the patient also take aspirin (acetylsalicylic acid).
- The doctor will decide how long the patient should receive Neoparin.
ST-segment elevation myocardial infarction (STEMI) in patients 75 years of age or older:
- The usual dose is 75 IU (0.75 mg) per kilogram of body weight every 12 hours.
- The maximum dose of Neoparin in the first two doses is 7500 IU (75 mg).
- The doctor will decide how long the patient should receive Neoparin.
Patients undergoing percutaneous coronary intervention (PCI):
Depending on when the last dose of Neoparin was administered, the doctor may decide to administer an additional dose of Neoparin before the PCI procedure. The medicine will be administered intravenously.
- 3. Prevention of blood clot formation in dialysis tubes
- The usual dose is 100 IU (1 mg) per kilogram of body weight.
- Neoparin is injected into the tube returning blood from the body (into the so-called arterial line) at the beginning of the dialysis session. This dose is usually sufficient for a 4-hour dialysis session. However, if necessary, the doctor may administer an additional dose of 50 IU to 100 IU (0.5 to 1 mg) per kilogram of body weight.
Instructions for using the pre-filled syringe
Method of self-administering Neoparin injection by the patient
If the patient can self-administer this medicine, the doctor or nurse will show them how to do it.
You should not attempt to self-administer the medicine without prior training.
If the patient is unsure what to do, they should consult their doctor or nurse immediately.
Before self-administering Neoparin
- Check the expiration date of the medicine. Do not use it if the date has expired.
- Check if the pre-filled syringe is damaged and if the medicine is a clear solution. If not, use another pre-filled syringe.
- Do not use this medicine if the patient notices any changes in the appearance of the product.
- Make sure the planned dose of the medicine is known.
- Examine the abdomen to check if the last injection did not cause redness, skin discoloration, swelling, discharge, or persistent pain
- if so, consult a doctor or nurse.
- Decide where the medicine will be injected. The medicine should be administered alternately on the left and right sides of the abdomen. The medicine should be injected just under the skin on the abdomen, but not too close to the navel or any scar tissue (at least 5 cm away).
- The pre-filled syringe is for single use only.
Instructions for self-administering Neoparin
- 1)Wash your hands and the injection site with water and soap. Dry.
- 2)Sit or lie down in a comfortable, relaxed position. Make sure the planned injection site is visible. It is ideal to use a recliner, armchair, or bed with pillows.
- 3)Choose a site on the right or left side of the abdomen. It should be at least 5 cm away from the navel towards the side.
Important:Do not inject the medicine into areas within 5 cm of the navel or around scars or bruises. The medicine should be injected alternately on the left and right sides of the abdomen, changing sides each time.
- 4)Carefully remove the needle cap. The pre-filled syringe is pre-filled with the medicine and ready for use.
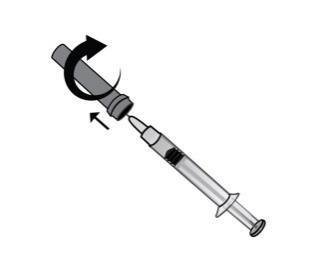
Do notpress the plunger before injecting the medicine to remove air bubbles.
This may cause loss of medicine. After removing the cap, do not allow the needle to come into contact with any object. This is to ensure the sterility of the needle.
- 5)Hold the pre-filled syringe (like a pencil) in the writing hand, and with the other hand, gently squeeze the cleaned area on the abdomen between the thumb and index finger, creating a skin fold.
Remember to hold the skin fold in this way throughout the injection.
- 6)Hold the pre-filled syringe so that the needle is facing downwards (at a 90º angle). Insert the entire length of the needle into the skin fold.
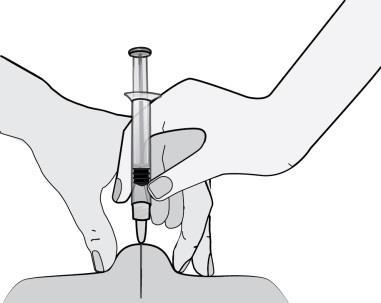
- 7)Press the plunger with your finger. This will cause the medicine to move into the fatty tissue on the abdomen. Remember to hold the skin fold throughout the injection.
- 8)Remove the needle, pulling it straight out.
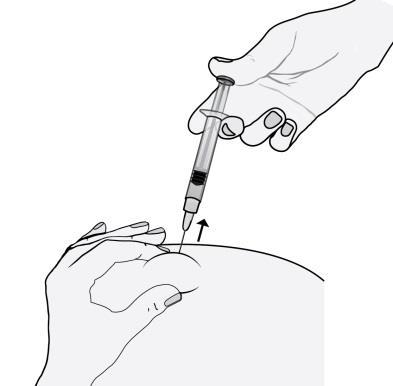
To avoid bruising, do not rub the injection site after administration.
- 9)Dispose of the used pre-filled syringe.
Pre-filled syringes should not be disposed of in household waste bins.
Change of anticoagulant medicine
- Change from Neoparin to vitamin K antagonist medicines (e.g., warfarin)The doctor will recommend blood tests to determine the INR (International Normalized Ratio) and, based on this, will inform the patient when to stop taking Neoparin.
- Change from vitamin K antagonist medicines (e.g., warfarin) to NeoparinThe patient should stop taking the vitamin K antagonist medicine. The doctor will recommend a blood test to determine the INR and, based on this, will inform the patient when to start taking Neoparin.
- Change from Neoparin to direct oral anticoagulantsThe patient should stop taking Neoparin. Then, they should start taking the direct oral anticoagulant 0-2 hours before the scheduled time of the next Neoparin injection. Then, they should continue taking the oral anticoagulant as usual.
- Change from direct oral anticoagulant to NeoparinThe patient should stop taking the direct oral anticoagulant. Neoparin treatment can be started 12 hours after the last dose of the direct oral anticoagulant.
Use in children and adolescents
The safety and efficacy of Neoparin in children and adolescents have not been evaluated.
Overdose of Neoparin
If the patient thinks they have used too much or too little Neoparin, they should immediately consult their doctor, nurse, or pharmacist, even if they do not notice any problems.
In case of accidental injection or ingestion of Neoparin by a child, they should immediately go to the hospital emergency department.
Missed dose of Neoparin
If a dose is missed, the patient should take it as soon as possible. They should not take a double dose to make up for the missed dose. Keeping a diary helps to ensure that no dose is missed.
Stopping Neoparin treatment
If the patient has any further doubts about using this medicine, they should consult their doctor, pharmacist, or nurse.
It is important to continue injecting Neoparin until the doctor recommends stopping it. If treatment is stopped, a blood clot may form, which can be very dangerous.
4. Possible side effects
Like all medicines, Neoparin can cause side effects, although not everybody gets them.
As with other similar medicines (used to reduce blood clotting), Neoparin can cause bleeding, which can potentially be life-threatening. In some cases, bleeding may not be immediately visible.
If the patient experiences any bleeding that does not stop on its own, as well as signs of excessive bleeding (severe weakness, fatigue, paleness, dizziness, headaches, or unexplained sweating), they should immediately consult their doctor.
The doctor may decide to monitor the patient more closely or change the medicine.
The patient should stop using Neoparin and immediately consult their doctor or nurse if they experience signs of a severe allergic reaction (such as difficulty breathing, swelling of the lips, mouth, throat, or eyes).
If the patient experiences any of the following symptoms, they should stop using enoxaparin and seek medical help immediately:
- A red, scaly, widespread rash with thickening of the skin and blisters, accompanied by fever. These symptoms usually appear at the beginning of treatment (acute generalized exanthematous pustulosis).
The patient should immediately consult their doctor:
- If they experience any signs of a blood clot blocking a blood vessel, such as:
- cramping pain, redness, increased warmth, or swelling in one of the lower limbs - these are symptoms of deep vein thrombosis
- shortness of breath, chest pain, fainting, or coughing up blood - these are symptoms of pulmonary embolism
- If the patient experiences painful rash or dark red spots under the skin that do not disappear when pressed. The doctor may order a blood test to check the platelet count.
Summary of possible side effects:
Very common (may affect more than 1 in 10 people)
- Bleeding.
- Increased liver enzyme activity.
Common (may affect up to 1 in 10 people)
- Increased tendency to bruise. This may be due to a decrease in platelet count.
- Pink spots on the skin. These changes are more likely to occur at the Neoparin injection sites.
- Skin rash (hives).
- Itchy, red skin.
- Bruising or pain at the injection site.
- Decreased red blood cell count.
- Increased platelet count.
- Headaches.
Uncommon (may affect up to 1 in 100 people)
- Sudden severe headache. This may be a sign of bleeding into the brain.
- Tenderness and swelling in the stomach. This may be a sign of bleeding into the stomach.
- Large, red, irregularly shaped skin changes with blisters or without blisters.
- Skin irritation (local irritation).
- The patient may notice yellowing of the skin or eyes and darker urine. This may indicate liver disease.
Rare (may affect up to 1 in 1000 people)
- Severe allergic reaction. Symptoms of such a reaction may include: rash, difficulty swallowing or breathing, swelling of the lips, face, throat, or tongue.
- Increased potassium levels in the blood. This is more likely in people with kidney disease or diabetes. The doctor can check this with a blood test.
- Increased eosinophil count in the blood. The doctor can check this with a blood test.
- Hair loss.
- Osteoporosis (a condition in which bones are more prone to fractures) after long-term use of the medicine.
- Numbness, tingling, and muscle weakness (especially in the lower part of the body) after lumbar puncture or spinal or epidural anesthesia.
- Loss of control over the bladder or bowels (a condition in which the patient cannot control when they need to go to the toilet).
- Hardening or a lump at the injection site.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych (Polish Medicines Agency)
Al. Jerozolimskie 181C
02-222 Warszawa
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Neoparin
Store in a temperature below 25°C. The medicine can also be stored in a refrigerator (2°C - 8°C) for no more than 1 month. Do not freeze.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and pre-filled syringe after EXP. The expiration date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Neoparin contains
- The active substance of the medicine is enoxaparin sodium
Neoparin, 2000 IU (20 mg)/0.2 ml:One pre-filled syringe of 0.2 ml contains 2000 IU (20 mg) of enoxaparin sodium.
Neoparin, 4000 IU (40 mg)/0.4 ml:One pre-filled syringe of 0.4 ml contains 4000 IU (40 mg) of enoxaparin sodium.
Neoparin, 6000 IU (60 mg)/0.6 ml:One pre-filled syringe of 0.6 ml contains 6000 IU (60 mg) of enoxaparin sodium.
Neoparin, 8000 IU (80 mg)/0.8 ml:One pre-filled syringe of 0.8 ml contains 8000 IU (80 mg) of enoxaparin sodium.
Neoparin, 10,000 IU (100 mg)/1 ml:One pre-filled syringe of 1 ml contains 10,000 IU (100 mg) of enoxaparin sodium.
- The other ingredients (excipients) are: water for injections.
What Neoparin looks like and contents of the pack
Pre-filled syringes with a needle, with a protective cap or without a cap, in blisters, in a cardboard box.
2 or 10 pre-filled syringes with a needle of 0.2 ml
2 or 10 pre-filled syringes with a needle of 0.4 ml
2 or 10 pre-filled syringes with a needle of 0.6 ml
2 or 10 pre-filled syringes with a needle of 0.8 ml
2 or 10 pre-filled syringes with a needle of 1.0 ml
Not all pack sizes may be marketed.
Marketing authorization holder and importer
SciencePharma spółka z ograniczoną odpowiedzialnością (SciencePharma Ltd.)
ul. Chełmska 30/34, 00-725 Warszawa, Poland
Date of last revision of the leaflet: December 2023
Other sources of information
Detailed information about this medicine is available on the website of the Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych (Polish Medicines Agency) http://www.urpl.gov.pl/pl
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterSciencePharma Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NeoparinDosage form: Solution, 12,000 IU (120 mg)/0.8 mlActive substance: enoxaparinPrescription requiredDosage form: Solution, 15,000 IU (150 mg)/mlActive substance: enoxaparinPrescription requiredDosage form: Solution, 2000 IU (20 mg)/0.2 mlActive substance: enoxaparin
Alternatives to Neoparin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Neoparin in España
Alternative to Neoparin in Ucrania
Online doctors for Neoparin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Neoparin – subject to medical assessment and local rules.














