
Clexane forte

Ask a doctor about a prescription for Clexane forte

How to use Clexane forte
Leaflet accompanying the packaging: patient information
Clexane Forte, 12,000 IU (120 mg)/0.8 ml, solution for injection
Clexane Forte, 15,000 IU (150 mg)/1 ml, solution for injection
Enoxaparin sodium
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Clexane Forte and what is it used for
- 2. Important information before using Clexane Forte
- 3. How to use Clexane Forte
- 4. Possible side effects
- 5. How to store Clexane Forte
- 6. Package contents and other information
1. What is Clexane Forte and what is it used for
Clexane Forte contains the active substance enoxaparin sodium. It belongs to a group of medicines called low molecular weight heparins or LMWH.
How Clexane Forte works
Clexane Forte works in two ways.
- 1) It prevents the growth of existing blood clots. This helps the body to dissolve existing blood clots, making them no longer harmful.
- 2) It prevents the formation of new blood clots in the patient's blood.
What Clexane Forte is used for
Clexane Forte can be used for:
- Treating blood clots that have already formed in the patient's blood.
- Preventing the formation of blood clots in the patient's blood in the following cases: before and after surgery, during short-term illness when the patient will be unable to move for some time, in patients who have developed blood clots in the circulating blood due to cancer, to further prevent the formation of new blood clots.
- Preventing the formation of blood clots in unstable angina (when insufficient blood is supplied to the heart muscle) or after a heart attack.
- Preventing the formation of clots in dialysis tubes (used in people with severe kidney function disorders).
2. Important information before using Clexane Forte
Do not use Clexane Forte if:
- the patient is allergic to:
o enoxaparin sodium or any of the other ingredients of this medicine (listed in section 6) o heparin or other low molecular weight heparins, such as nadroparin, tinzaparin, or dalteparin. Symptoms of an allergic reaction may include: rash, difficulty breathing or swallowing, swelling of the face, lips, tongue, mouth, throat, or eyes.
- the patient has had a reaction to heparin that caused a significant decrease in the number of blood cells responsible for blood clotting (platelets) in the last 100 days.
- there are antibodies against enoxaparin in the patient's blood.
- the patient has severe bleeding or a medical condition associated with an increased risk of bleeding, such as: o stomach ulcers, recent brain or eye surgery, or recent hemorrhagic stroke.
- the patient is using Clexane Forte to treat blood clots and a lumbar puncture or surgery under spinal or epidural anesthesia is planned within 24 hours: o the patient should not use Clexane Forte. In case of doubts, consult a doctor or pharmacist before starting Clexane Forte.
Warnings and precautions
Clexane Forte should not be exchanged with other low molecular weight heparins such as nadroparin, tinzaparin, or dalteparin. This is because they are not exactly the same, differ in activity, and have different instructions for use. Before starting Clexane Forte, consult a doctor or pharmacist if:
- the patient has ever had a reaction to heparin that caused a significant decrease in the number of blood cells responsible for blood clotting (platelets)
- the patient has a heart valve replacement
- the patient has endocarditis (infection of the membrane lining the heart)
- the patient has stomach ulcers
- the patient has recently had a brain stroke
- the patient has high blood pressure
- the patient has diabetes or has problems with blood vessels in the eyes caused by diabetes (so-called diabetic retinopathy)
- the patient has recently had eye or brain surgery
- the patient is elderly (over 65 years), especially if they are over 75 years old
- the patient has kidney disease
- the patient has liver disease
- the patient is underweight or overweight
- the patient has an increased level of potassium in the blood (which can be checked with a blood test)
- the patient is currently taking medications that may cause bleeding (see section 2 "Clexane Forte and other medicines")
- the patient has spinal problems or has had spinal surgery. If any of the above situations apply to the patient or they have doubts, consult a doctor or pharmacist before starting Clexane Forte.
In the case of patients taking doses higher than 210 mg/day, this medicine contains more than 24 mg of sodium (the main component of table salt) in each dose. This corresponds to 1.2% of the maximum recommended daily sodium intake in the diet for adults.
Examinations and monitoring
Before starting this medicine and periodically during its use, the patient may undergo a blood test to check the number of platelets responsible for blood clotting and potassium levels in the blood.
Use in children and adolescents
The safety and efficacy of Clexane Forte have not been evaluated in children and adolescents.
Clexane Forte and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
- warfarin - a medicine used to thin the blood
- aspirin (also known as acetylsalicylic acid or ASA), clopidogrel, or other medicines used to prevent blood clots (see section 3 "Change of anticoagulant medicine")
- dextran injections - used as a blood substitute
- ibuprofen, diclofenac, ketorolac, or other non-steroidal anti-inflammatory medicines used to treat pain and swelling in arthritis and other conditions
- prednisolone, dexamethasone, or other medicines used to treat asthma, rheumatoid arthritis, and other conditions
- medicines that increase potassium levels in the blood, such as potassium salts, diuretics, or certain heart medicines.
Surgical procedures and anesthetics
If the patient is scheduled for a lumbar puncture or surgery under spinal or epidural anesthesia, they should inform their doctor that they are using Clexane Forte. See the section "When not to use Clexane Forte".
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine. In pregnant women with a mechanical heart valve, there may be an increased risk of blood clots. The doctor should discuss this with the patient. Women who are breastfeeding or plan to breastfeed should consult their doctor before starting this medicine.
Driving and using machines
Clexane Forte does not affect the ability to drive or use machines. It is recommended that the doctor documents the trade name and batch number of the product used.
3. How to use Clexane Forte
This medicine should always be used as directed by your doctor or pharmacist. If you have any doubts, consult your doctor or pharmacist.
Taking the medicine
- Clexane Forte will usually be administered to the patient by a doctor or nurse. This is because it requires injection.
- Clexane Forte is usually administered subcutaneously.
- Clexane Forte may be administered intravenously after certain types of heart attack or after surgery.
- Clexane Forte may be introduced into the dialysis tube returning blood from the body (into the so-called arterial line) at the beginning of the dialysis session.
- Clexane Forte should not be administered intramuscularly.
Dose
- The doctor will decide what dose of Clexane Forte the patient should take. The dose depends on the reason for using the medicine.
- In patients with kidney disease, the patient may receive a lower dose of Clexane Forte.
- 1) Treatment of blood clots in the patient's blood
- The usual dose is 150 IU (1.5 mg) per kilogram of body weight once daily or 100 IU (1 mg) per kilogram of body weight twice daily.
- The doctor will decide how long the patient should receive Clexane Forte.
- 2) Prevention of blood clot formation in the patient's blood during surgery or during periods of limited mobility due to illness
- The dose depends on the patient's risk of blood clot formation. The patient will receive Clexane Forte at a dose of 2000 IU (20 mg) or 4000 IU (40 mg) daily.
- In the case of planned surgery, the first injection is usually performed 2 hours or 12 hours before surgery.
- If the patient has limited mobility due to illness, they usually receive Clexane Forte at a dose of 4000 IU (40 mg) daily.
- The doctor will decide how long the patient should receive Clexane Forte.
- 3) Prevention of blood clot formation in patients with unstable angina or after a heart attack
- Clexane Forte can be used in two different types of heart attacks.
- The dose of Clexane Forte will depend on the patient's age and the type of heart attack that occurred.
Non-ST-segment elevation myocardial infarction (NSTEMI):
- The usual dose is 100 IU (1 mg) per kilogram of body weight every 12 hours.
- The doctor will usually recommend that the patient also take aspirin (acetylsalicylic acid).
- The doctor will decide how long the patient should receive Clexane Forte.
ST-segment elevation myocardial infarction (STEMI) in patients under 75 years of age:
- The initial dose of Clexane Forte is 3000 IU (30 mg) administered intravenously.
- Clexane Forte is also administered subcutaneously. The usual dose is 100 IU (1 mg) per kilogram of body weight every 12 hours.
- The doctor will usually recommend that the patient also take aspirin (acetylsalicylic acid).
- The doctor will decide how long the patient should receive Clexane Forte.
ST-segment elevation myocardial infarction (STEMI) in patients 75 years of age or older:
- The usual dose is 75 IU (0.75 mg) per kilogram of body weight every 12 hours.
- The maximum dose of Clexane Forte in the first two doses is 7500 IU (75 mg).
- The doctor will decide how long the patient should receive Clexane Forte.
Patient undergoing percutaneous coronary intervention (PCI):
- Depending on when the last dose of Clexane Forte was administered, the doctor may decide to administer an additional dose of Clexane Forte before the PCI procedure. The medicine will be administered intravenously.
- 4) Prevention of blood clot formation in dialysis tubes
- The usual dose is 100 IU (1 mg) per kilogram of body weight.
- Clexane Forte is injected into the tube returning blood from the body (into the so-called arterial line) at the beginning of the dialysis session. This dose usually lasts for a 4-hour dialysis session. However, if necessary, the doctor may administer an additional dose of 50 IU to 100 IU (0.5 to 1 mg) per kilogram of body weight.
Self-administration of Clexane Forte.
If the patient is able to self-administer Clexane Forte, the doctor or nurse will demonstrate how to do it. Do not attempt to self-administer if you have not been trained. If you have any doubts, consult your doctor or nurse immediately. Proper subcutaneous injection (so-called "subcutaneous injection") can reduce pain and bruising at the injection site.
Before self-administering Clexane Forte
- Prepare all necessary items: syringe, alcohol swab, soap and water, and a container for medical waste.
- Check the expiration date on the packaging. Do not use the medicine after the expiration date.
- Check if the syringe is damaged and if the liquid is clear. If not, use another syringe.
- Make sure what dose is to be administered.
- Examine the abdomen to check if the last injection has caused redness, skin color change, swelling, discharge, or if it is still painful. If so, consult a doctor or nurse.
Instructions for self-administering Clexane Forte: (Instructions for pre-filled syringes without a safety system)
Preparing the injection site
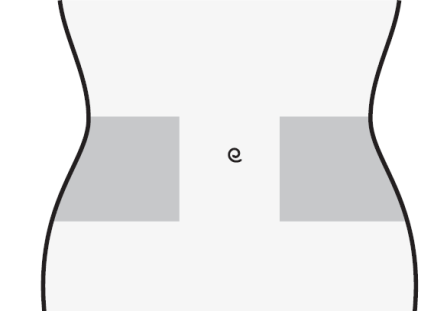
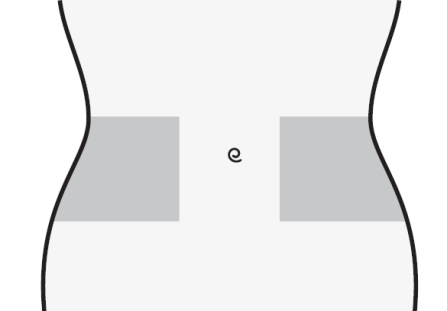
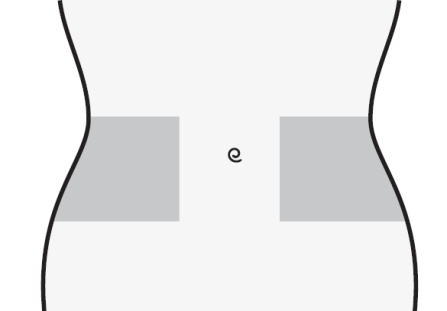
- 1) Choose an injection site on the right or left side of the abdomen. The injection site should be at least 5 cm away from the navel towards the sides.
- Do not inject within 5 cm of the navel or around existing scars or bruises.
- Change the injection site between the left and right side of the abdomen, depending on the site of the previous injection.
- 2) Wash your hands. Clean the injection site with an alcohol swab or soap and water.
- 3) Sit or lie down in a comfortable position, so you are relaxed. Make sure the injection site is within your sight. A chair, couch, or bed with pillows for support will be suitable.
Dose selection
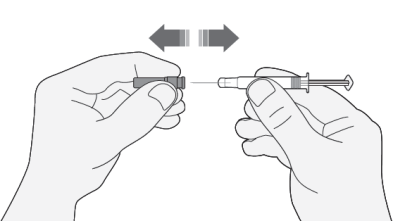
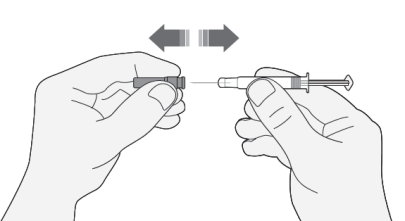
- 1) Carefully remove the needle cap from the syringe. Discard the cap.
- Do not press the plunger before injecting to remove air bubbles. This may reduce the administered dose.
- After removing the cap, do not touch the needle. This will ensure the sterility of the needle.
- 2) If the amount of medicine in the syringe is consistent with the prescribed dose, there is no need to adjust the dose. You can now perform the injection.
- 3) If the dose depends on body weight, it may be necessary to adjust the dose in the syringe according to the prescribed dose. In this case, remove excess medicine by holding the syringe upside down (to maintain air bubbles in the syringe) and discard the excess into a container.
- 4) A drop may appear at the tip of the needle. If this happens, remove the drop before injecting by tapping the syringe with the needle facing down. You can now perform the injection.
Injection
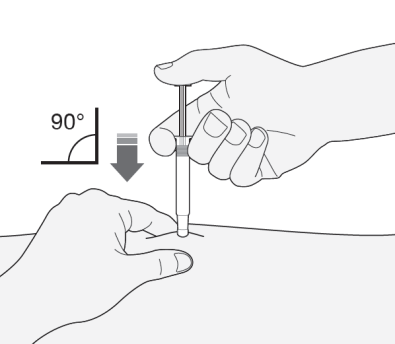
- 1) Hold the syringe in the hand you write with (like a pencil). With your other hand, gently grasp the cleaned abdominal skin with your index finger and thumb, creating a skin fold between your fingers.
- Make sure to maintain the skin fold during the injection.
- 2) Hold the syringe with the needle facing down (perpendicularly at a 90° angle). Insert the entire length of the needle into the skin fold.
- 3) Press the plunger with your thumb. This will introduce the medicine into the abdominal fatty tissue. Administer the entire amount of medicine from the syringe.
- 4) Remove the needle from the injection site, pulling it straight out. Keep the needle away from yourself and others. You can now release the skin fold.
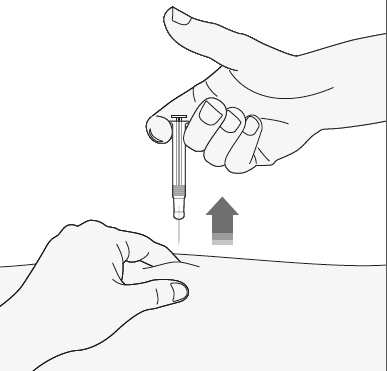
After the injection
- 1) To avoid bruising, do not rub the injection site after the injection.
- 2) Dispose of the used syringe in a container for medical waste. Close the container and store it in a place that is not visible and inaccessible to children. If the container is full, dispose of it according to the doctor's or pharmacist's instructions.
Dispose of any unused medicines or waste in accordance with local regulations.
(Instructions for pre-filled syringes with a safety system of the ERIS type)
Preparing the injection site
- 1) Choose an injection site on the right or left side of the abdomen. The injection site should be at least 5 cm away from the navel towards the sides.
- Do not inject within 5 cm of the navel or around existing scars or bruises.
- Change the injection site between the left and right side of the abdomen, depending on the site of the previous injection.
- 2) Wash your hands. Clean the injection site with an alcohol swab or soap and water.
- 3) Sit or lie down in a comfortable position, so you are relaxed. Make sure the injection site is within your sight. A chair, couch, or bed with pillows for support will be suitable.
Dose selection
- 1) Carefully remove the needle cap from the syringe. Discard the cap.
- Do not press the plunger before injecting to remove air bubbles. This may reduce the administered dose.
- After removing the cap, do not touch the needle. This will ensure the sterility of the needle.
- 2) If the amount of medicine in the syringe is consistent with the prescribed dose, there is no need to adjust the dose. You can now perform the injection.
- 3) If the dose depends on body weight, it may be necessary to adjust the dose in the syringe according to the prescribed dose. In this case, remove excess medicine by holding the syringe upside down (to maintain air bubbles in the syringe) and discard the excess into a container.
- 4) A drop may appear at the tip of the needle. If this happens, remove the drop before injecting by tapping the syringe with the needle facing down. You can now perform the injection.
Injection
- 1) Hold the syringe in the hand you write with (like a pencil). With your other hand, gently grasp the cleaned abdominal skin with your index finger and thumb, creating a skin fold between your fingers.
- Make sure to maintain the skin fold during the injection.
- 2) Hold the syringe with the needle facing down (perpendicularly at a 90° angle). Insert the entire length of the needle into the skin fold.
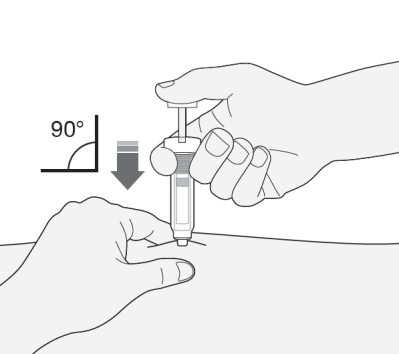
- 3) Press the plunger with your thumb. This will introduce the medicine into the abdominal fatty tissue. Administer the entire amount of medicine from the syringe.
- 4) Remove the needle from the injection site, pulling it straight out. The protective shield will automatically cover the needle. You can now release the skin fold. The safety system will release the protective shield only when the syringe is emptied by pressing the plunger to the end.
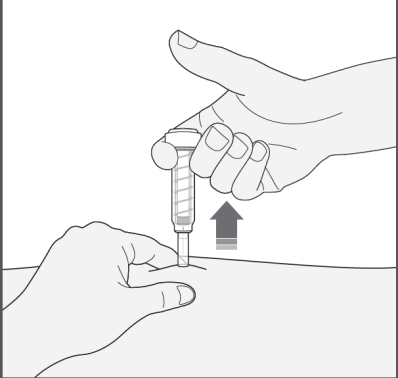
After the injection
- 1) To avoid bruising, do not rub the injection site after the injection.
- 2) Dispose of the used syringe in a container for medical waste. Close the container and store it in a place that is not visible and inaccessible to children. If the container is full, dispose of it according to the doctor's or pharmacist's instructions.
Dispose of any unused medicines or waste in accordance with local regulations.
(Instructions for pre-filled syringes with a safety system of the PREVENTIS type)
Preparing the injection site
- 1) Choose an injection site on the right or left side of the abdomen. The injection site should be at least 5 cm away from the navel towards the sides.
- Do not inject within 5 cm of the navel or around existing scars or bruises.
- Change the injection site between the left and right side of the abdomen, depending on the site of the previous injection.
- 2) Wash your hands. Clean the injection site with an alcohol swab or soap and water.
- 3) Sit or lie down in a comfortable position, so you are relaxed. Make sure the injection site is within your sight. A chair, couch, or bed with pillows for support will be suitable.
Dose selection
- 1) Carefully remove the needle cap from the syringe. Discard the cap.
- Do not press the plunger before injecting to remove air bubbles. This may reduce the administered dose.
- After removing the cap, do not touch the needle. This will ensure the sterility of the needle.
- 2) If the amount of medicine in the syringe is consistent with the prescribed dose, there is no need to adjust the dose. You can now perform the injection.
- 3) If the dose depends on body weight, it may be necessary to adjust the dose in the syringe according to the prescribed dose. In this case, remove excess medicine by holding the syringe upside down (to maintain air bubbles in the syringe) and discard the excess into a container.
- 4) A drop may appear at the tip of the needle. If this happens, remove the drop before injecting by tapping the syringe with the needle facing down. You can now perform the injection.
Injection
- 1) Hold the syringe in the hand you write with (like a pencil). With your other hand, gently grasp the cleaned abdominal skin with your index finger and thumb, creating a skin fold between your fingers.
- Make sure to maintain the skin fold during the injection.
- 2) Hold the syringe with the needle facing down (perpendicularly at a 90° angle). Insert the entire length of the needle into the skin fold.
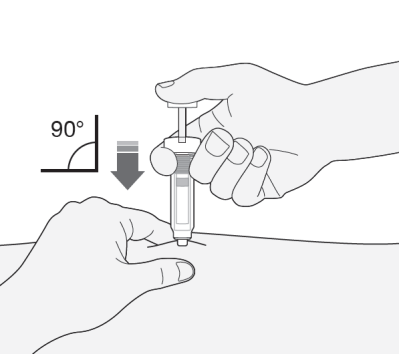
- 3) Press the plunger with your thumb. This will introduce the medicine into the abdominal fatty tissue. Administer the entire amount of medicine from the syringe.
- 4) Remove the needle from the injection site, pulling it straight out while still holding the plunger. Keep the needle away from yourself and others. Firmly press the plunger to activate the safety system. The protective shield will automatically cover the needle. A audible "click" will confirm the activation of the protective shield. You can now release the skin fold.
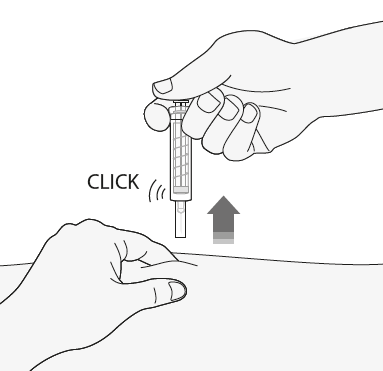
After the injection
- 1) To avoid bruising, do not rub the injection site after the injection.
- 2) Dispose of the used syringe in a container for medical waste. Close the container and store it in a place that is not visible and inaccessible to children. If the container is full, dispose of it according to the doctor's or pharmacist's instructions. Dispose of any unused medicines or waste in accordance with local regulations.
Change of anticoagulant medicine
- Change from Clexane Forte to medicines that thin the blood, called vitamin K antagonists (such as warfarin)The doctor will recommend that the patient undergo blood tests to determine the INR ratio and inform the patient when to stop taking Clexane Forte.
- Change from vitamin K antagonists (such as warfarin) to Clexane ForteStop taking the vitamin K antagonist. The doctor will recommend that the patient undergo blood tests to determine the INR ratio and inform the patient when to start taking Clexane Forte.
- Change from Clexane Forte to direct oral anticoagulantsStop taking Clexane Forte. Then start taking the direct oral anticoagulant 0 to 2 hours before the scheduled time of the next injection; and then continue taking the medicine as usual.
- Change from direct oral anticoagulant to Clexane Forte
Clexane Forte
Stop taking the direct oral anticoagulant. Clexane Forte treatment can be started only after 12 hours have passed since the last dose of the direct oral anticoagulant.
Use of a higher dose of Clexane Forte than recommended
If the patient thinks they have used too much or too little Clexane Forte, they should immediately inform their doctor, pharmacist, or nurse, even if they do not notice any problems. In case of accidental injection or ingestion of Clexane Forte by a child, immediately go to the hospital emergency department.
Missing a dose of Clexane Forte
If a dose is missed, take it as soon as possible. Do not take a double dose to make up for the missed dose. Keeping a diary helps ensure that no dose is missed.
Stopping Clexane Forte
It is essential to continue injecting Clexane Forte until the doctor recommends stopping it. If treatment is stopped, a blood clot may form, which can be very dangerous. If you have any further doubts about using this medicine, consult your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Clexane Forte can cause side effects, although not everybody gets them.
Severe side effects
Stop using Clexane Forte and consult a doctor or nurse immediately if:
you experience symptoms of a severe allergic reaction (such as rash, difficulty breathing or swallowing, swelling of the face, lips, tongue, mouth, throat, or eyes).
If you experience any of the following symptoms, stop using enoxaparin and seek medical attention immediately:
- A red, scaly, widespread rash with thickening of the skin and blisters, accompanied by fever. Symptoms usually appear at the beginning of treatment (acute generalized exanthematous pustulosis).
Like other similar medicines used to reduce blood clotting, Clexane Forte can cause bleeding. This can be life-threatening. In some cases, bleeding may not be immediately visible.
Consult a doctor immediately if:
- the patient experiences any bleeding that does not stop on its own
- the patient shows signs of excessive bleeding, such as weakness, fatigue, paleness, dizziness with headaches, or swelling of unknown origin. The doctor may decide to monitor the patient more closely or change the medicine.
Consult a doctor immediately:
- if the patient experiences any signs of a blood clot, such as: o severe, crampy pain, redness, increased warmth, or swelling in one leg - these are symptoms of deep vein thrombosis o shortness of breath, chest pain, fainting, or coughing up blood - these are symptoms of pulmonary embolism
- if the patient experiences painful rash or dark red spots under the skin that do not fade when pressed. The doctor may order blood tests to check the platelet count.
Other side effects:
Very common(may affect more than 1 in 10 people):
- bleeding
- increased liver enzyme activity.
Common(may affect up to 1 in 10 people):
- increased tendency to bruise. This may be due to a decrease in platelet count
- red spots on the skin. These changes are more likely at the injection sites of Clexane Forte
- skin rash (hives)
- itching, red skin
- bruising or pain at the injection site
- decreased red blood cell count
- increased platelet count
- headaches.
Uncommon(may affect up to 1 in 100 people):
- sudden, severe headache - may be a sign of bleeding in the brain
- feeling of tenderness and swelling in the stomach - may be a sign of bleeding in the stomach
- large, red skin changes with an irregular shape, with or without blisters
- skin irritation (local irritation)
- the patient may notice yellowing of the skin or eyes and darker urine. This may indicate liver disease.
Rare(may affect up to 1 in 1000 people):
- severe allergic reaction - symptoms may include: rash, difficulty swallowing or breathing, swelling of the lips, face, throat, or tongue
- increased potassium levels in the blood - this is more likely in people with kidney disease or diabetes. The doctor may check this with a blood test
- increased eosinophil count in the blood - the doctor may check this with a blood test
- hair loss
- osteoporosis (a condition in which bones are more prone to fractures) after long-term use of the medicine
- tingling, numbness, and weakness of the muscles (especially in the lower part of the body) after a lumbar puncture or spinal or epidural anesthesia
- loss of control over the bladder or bowels (a condition in which the patient is unable to control when they need to go to the toilet)
- hardening or a lump at the injection site.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw. Tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder or its representative in Poland. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Clexane Forte
Do not store above 25°C. Do not freeze. Store in a place that is not visible and inaccessible to children. Do not use this medicine after the expiration date stated on the packaging. The expiration date refers to the last day of the specified month. Do not use this medicine if you notice that the syringe is cracked, there are solid particles in the solution, or the solution has an unusual color (see "What Clexane Forte looks like and what the pack contains"). Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Clexane Forte contains
- The active substance is enoxaparin sodium.
- Each ml contains 150 mg of enoxaparin sodium, which corresponds to 15,000 IU of anti-Xa activity. Each 0.8 ml pre-filled syringe contains 12,000 IU (120 mg) of enoxaparin sodium. Each 1 ml pre-filled syringe contains 15,000 IU (150 mg) of enoxaparin sodium.
- The other ingredient is water for injections.
What Clexane Forte looks like and what the pack contains
Clexane Forte is a clear, colorless or pale yellow solution for injection in a glass pre-filled syringe (with or without an automatic safety system). Pack sizes: 2, 5, 6, 10, 20, 30, 50 pre-filled syringes and in multipacks of 3 x 10 pre-filled syringes. Not all pack sizes may be marketed.
Marketing authorization holder
Sanofi Winthrop Industrie 82, Avenue Raspail 94250 Gentilly France
Manufacturer
Sanofi Winthrop Industrie 180, rue Jean Jaurès 94 700 Maisons-Alfort France Sanofi-Aventis Private Co. Ltd Budapest Logistics and Distribution Platform Bdg. DC5, Campona utca1. Budapest, 1225 Hungary Sanofi-Aventis GmbH Turm A, 29. OG, Wienerbergstraße 11 1100 Vienna Austria Sanofi Winthrop Industrie 1051 Boulevard Industriel 76580 Le Trait France Sanofi-Aventis Deutschland GmbH Industriepark Höchst-Brüningstraße 50 65926 Frankfurt am Main Germany
To obtain more detailed information on the medicine and its names in the Member States of the European Economic Area, contact the representative of the marketing authorization holder in Poland:
Sanofi Sp. z o.o. ul. Marcina Kasprzaka 6 01-211 Warsaw tel.: +48 22 280 00 00
Date of last revision of the leaflet:
Other sources of information
Detailed information on this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products (www.urpl.gov.pl).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterSanofi Winthrop Industrie Sanofi Winthrop Industrie Sanofi-Aventis Deutschland GmbH Sanofi-Aventis GmbH Sanofi-Aventis Private Co. Ltd.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Clexane forteDosage form: Solution, 12,000 IU (120 mg)/0.8 mlActive substance: enoxaparinPrescription requiredDosage form: Solution, 2000 IU (20 mg)/0.2 mlActive substance: enoxaparinDosage form: Solution, 4000 IU (40 mg)/0.4 mlActive substance: enoxaparin
Alternatives to Clexane forte in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Clexane forte in Spain
Alternative to Clexane forte in Ukraine
Online doctors for Clexane forte
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Clexane forte – subject to medical assessment and local rules.














