Nebbud

Ask a doctor about a prescription for Nebbud

How to use Nebbud
Leaflet accompanying the packaging: patient information
Nebbud, 0.25 mg/2 ml, nebuliser suspension
Budesonide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Nebbud and what is it used for
- 2. Important information before using Nebbud
- 3. How to use Nebbud
- 4. Possible side effects
- 5. How to store Nebbud
- 6. Contents of the packaging and other information
1. What is Nebbud and what is it used for
Budesonide belongs to a group of medicines called glucocorticosteroids, which are used to reduce or prevent inflammatory reactions (swelling) in the lungs.
This medicine can be used in adults, adolescents, children, and infants from 6 months of age and older.
This medicine is used to treat asthma in patients for whom the use of other types of inhalers, such as pressurized inhalers or powder inhalers, is unsatisfactory or inappropriate.
Nebbud is also used in the hospital treatment of infants and children with severe croup or acute laryngitis (laryngitis subglottica).
Nebbud can also be used to treat pulmonary exacerbations in chronic obstructive pulmonary disease (COPD) as an alternative to systemic (oral and/or injectable) anti-inflammatory medications, only after proper training in the use of this medicine in nebulization and for no longer than 10 consecutive days.
2. Important information before using Nebbud
When not to use Nebbud
- if the patient is allergic to budesonide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
If any of the following conditions apply to the patient, they should inform their doctor before starting to use Nebbud:
- if the patient has or has had tuberculosis;
- if the patient currently has or has had liver disease;
- if the patient has a fungal, viral, or other infection of the respiratory tract, such as a cold or chest infection;
- if the patient experiences symptoms such as muscle and joint pain, fatigue, headache, nausea, and vomiting. These symptoms may occur when switching from oral corticosteroid tablets (such as prednisolone) to Nebbud. The doctor will adjust the treatment if necessary;
- if the patient is switching from oral corticosteroid tablets to Nebbud nebuliser suspension, an allergy may be revealed. This is because oral anti-inflammatory tablets suppress the symptoms of an allergy;
- if the patient experiences blurred vision or other vision disturbances, they should consult a doctor.
Nebbud is not suitable for use in acute bronchospasm. Acute bronchospasm should be treated with a short-acting bronchodilator. If shortness of breath and wheezing occur immediately after administration of Nebbud, the use of the medicine should be stopped immediately and a doctor consulted.
Budesonide is a steroid. It should be noted that when using this medicine, a positive result may be obtained in doping tests. In case of any doubts, consult a doctor.
Children and adolescents
In rare cases, during long-term treatment with budesonide, growth retardation may occur in children and adolescents. In the case of long-term use of the medicine in a child, the doctor will regularly monitor their growth.
After each inhalation, the mouth should be rinsed with water. This reduces the risk of oral thrush and hoarseness. If fungal infections of the mouth (white spots on the tongue or mouth) or hoarseness occur, the doctor should be informed.
Inhaling the medicine through a mouthpiece instead of a face mask reduces the risk of skin irritation. If a face mask is used, the face should be washed immediately after inhalation. Alternatively, the skin covered by the mask can be rubbed with petroleum jelly before inhalation (and washed off after inhalation).
In the case of long-term use of higher doses than recommended, symptoms may occur that can also be observed when using oral anti-inflammatory tablets, such as a "full face".
Nebbud and other medicines
The doctor or pharmacist should be informed about all medicines the patient is currently taking or has recently taken, as well as medicines that are available without a prescription.
Some medicines may enhance the effect of Nebbud, and the doctor may want to closely monitor the patient's condition when taking such medicines (including some HIV medicines: ritonavir, cobicistat):
- medicines used to treat fungal infections, such as ketoconazole or itraconazole
- antibiotics, erythromycin, and clarithromycin
- other medicines that facilitate breathing
- estrogens and contraceptive steroids.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
Pregnancy
There is no evidence that the use of Nebbud during pregnancy is harmful to the mother or child.
It is essential to properly treat asthma during pregnancy. The worsening of the disease can be harmful to both the mother and the unborn child. To control asthma, the smallest possible dose of Nebbud should be used. Therefore, it is recommended to consult a doctor in case of pregnancy during treatment with Nebbud.
Breastfeeding
There is no evidence that the use of Nebbud during breastfeeding is harmful to the mother or child. If the patient is breastfeeding, they do not need to stop using Nebbud.
Driving and using machines
Nebbud has no influence or negligible influence on the ability to drive or use machines.
3. How to use Nebbud
This medicine should always be used as directed by the doctor. In case of doubts, consult a doctor or pharmacist.
The doctor will determine the dose of the medicine suitable for the patient, depending on the severity of asthma.
Improvement in health may occur as early as 3 days after starting to use the medicine; however, the full therapeutic effect may occur within 2 to 4 weeks. It is essential to take the medicine as directed by the doctor, even if the patient feels better.
The recommended doses of the medicine are as follows:
Asthma
Adults (including elderly patients) and adolescents from 12 years of age
The usual dose is 0.5 to 2 mg of budesonide per day. The daily dose is usually divided into two doses taken throughout the day. If asthma is stable and not severe, the doctor may recommend using this medicine once a day. The doctor will inform the patient how and when to take the medicine. The doctor's instructions should always be followed.
Infants and children (from 6 months to 11 years of age)
The usual dose is 0.25 mg to 1 mg of budesonide per day. The doctor will inform how the child should take this medicine. It is usually recommended to take the medicine in two doses at different times throughout the day. If asthma is stable and not severe, the doctor may recommend using the medicine once a day.
Croup
The usual dose in infants and children is 2 mg of budesonide per day. This dose can be given as a whole (2 ampoules of 1 mg/2 ml) or divided into two doses and given as two 1 mg ampoules at 30-minute intervals. This dosing regimen can be repeated every 12 hours, up to 36 hours, or until the patient's condition improves.
Exacerbations in chronic obstructive pulmonary disease (COPD)
The recommended dose of budesonide is 4 to 8 mg per day, divided into 2 to 4 doses. Nebbud should be used until the symptoms resolve, but for no longer than 10 consecutive days.
Instructions for using the medicine
The medicine should be used with a jet nebuliser. The resulting "mist" is then inhaled through a mouthpiece or face mask. Ultrasonic nebulisers should not be used to administer this medicine.
To take the medicine, follow these steps:
- 1. Separate one sterile plastic container (ampoule) from the strip by twisting and pulling (Figure A).
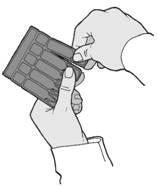
(Figure A).
- 2. Gently shake the ampoule in a circular motion for about 10 seconds or until the sediment is no longer visible.
- 3. Holding the ampoule vertically, unscrew the top part of the ampoule (Figure B).
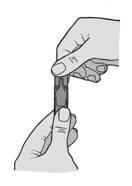
(Figure B).
- 4. Squeeze the entire contents of the ampoule into the nebuliser chamber (Figure C). Put the cover on the nebuliser chamber and carefully remove the empty ampoule.
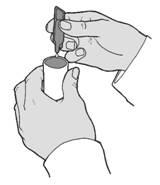
(Figure C)
- 5. Connect one end of the nebuliser chamber to the mouthpiece or face mask and the other end to the air pump.
- 6. Gently shake the nebuliser chamber and then turn on the nebuliser. Breathe in the "mist" calmly and deeply through the mouthpiece or face mask.
- 7. When no more "mist" appears in the mouthpiece or face mask, the inhalation is complete.
- 8. Rinse the mouth with water. Spit out the water. Do not swallow. If a face mask is used, the face should also be washed. It is essential to do this, as it may reduce the risk of certain side effects associated with this medicine.
- 9. After each use, clean the nebuliser. Wash the nebuliser container and mouthpiece or face mask in warm water with a mild detergent, following the manufacturer's instructions. The nebuliser should be carefully rinsed and dried by connecting the nebuliser chamber to the air inlet of the air pump.
It is essential to always follow the manufacturer's instructions, which are attached to the nebuliser.
If the patient has doubts about how to use the nebuliser, they should talk to a doctor or pharmacist.
The doctor may also recommend the following:
- In periods of stress (e.g., during infection) or when the patient is taking high doses of inhaled steroids for a long time, or before surgery, the doctor may consider additional treatment with oral steroids.
- If the patient with asthma has been taking oral steroids, the doctor may reduce the number of tablets taken after the patient starts using Nebbud nebuliser suspension. As a result, symptoms such as a stuffy nose or cold, lack of energy, depression, rash (a type of skin rash), and joint or muscle pain may occur. If any of these symptoms bother the patient or do not go away, they should consult a doctor.
- The doctor may recommend mixing this medicine with 0.9% sodium chloride solution or solutions containing other active substances that act on the respiratory tract, such as salbutamol, terbutaline, sodium cromoglicate, and ipratropium bromide. In such cases, the instructions should be followed carefully. The mixture should be used within 30 minutes of preparation. The medicine should not be mixed with other medicines unless recommended by the doctor.
Using a higher dose of Nebbud than recommended
Consult a doctor or pharmacist as soon as possible. Take the packaging of the medicine with all the remaining ampoules. It is essential to take the dose of the medicine as indicated in the leaflet or as recommended by the doctor. Do not increase or decrease the dose of the medicine without consulting a doctor.
Missing a dose of Nebbud
Do not take a double dose to make up for a missed dose. Take the next dose at the usual time.
In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Nebbud can cause side effects, although not everybody gets them.
All medicines can cause allergic reactions, but severe allergic reactions are very rare.
Inform the doctor immediately if the patient experiences any of the following symptoms:
sudden wheezing, difficulty breathing, swelling of the eyelids, face, or lips, rash, or itching (especially if it affects the whole body).
Rarely, inhaled medicines like budesonide can cause sudden wheezing and/or shortness of breath.
If any of these symptoms occur, stop using the medicine immediately and consult a doctor.
The following side effects have been reported:
Common side effects(occurring in less than 1 in 10 people):
Pain and/or irritation of the mouth (including thrush), throat irritation, hoarseness, difficulty swallowing, cough.
Pneumonia (lung infection) in patients with COPD
Inform the doctor if any of the following symptoms occur while using budesonide; these may be symptoms of a lung infection:
- fever or chills,
- increased production of sputum, change in sputum color,
- worsening cough or increased difficulty breathing.
Uncommon side effects(occurring in less than 1 in 100 people):
Anxiety
Depression
Aggression
Hyperactivity
Sleep problems
Tremors
Clouding of the lens in the eye (cataract, glaucoma)
Muscle cramps
Blurred vision
Rare side effects(occurring in less than 1 in 1000 people):
Skin reactions, including itching, rash, bruising, inflammation, redness of the skin, and/or skin eruptions, swelling, growth retardation in children and adolescents, hypersensitivity (allergy to the medicine), and bronchospasm (muscle tension in the airways causing wheezing).
Adrenal insufficiency (reduced activity of the adrenal gland) may also occur. The main symptoms of adrenal insufficiency are headaches, fatigue, nausea, and vomiting, weight loss, abdominal pain, and loss of appetite.
Anxiety, nervousness, and irritability (more likely to occur in children).
Change in voice.
Very rare side effects(occurring in less than 1 in 10,000 people):
Decreased bone density (thinning of the bones).
Side effects with unknown frequency (frequency cannot be estimated from the available data):
Glaucoma (increased pressure in the eye).
Reporting side effects
If side effects occur, including those not listed in this leaflet, inform the doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Nebbud
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the outer packaging, sachet, and ampoule after EXP. The expiry date refers to the last day of the month.
Do not freeze.
Store the medicine in an upright position.
After opening the sachet, the ampoules inside should be used within 3 months (it is worth noting the opening date on the foil sachet). After opening the sachet, the ampoules should be stored in the sachet, in the carton, to protect them from light. They should not be frozen.
Each ampoule is for single use only.
After opening the ampoule: use immediately. Any unused medicine should be disposed of.
Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Nebbud contains
- The active substance of the medicine is budesonide.
Nebbud, 0.25 mg/2 ml, nebuliser suspension contains 0.25 mg of budesonide as the active substance in each single-dose ampoule of 2 ml.
- The other ingredients of the medicine are: disodium edetate, sodium chloride, polysorbate 80, citric acid monohydrate, sodium citrate, and water for injections.
What Nebbud looks like and contents of the packaging
The medicine is available in plastic, single-dose ampoules containing 2 ml of white or almost white, sterile nebuliser suspension (transformed into a fine mist for inhalation).
Sets of 5 ampoules made of LDPE in a sachet of PET/LDPE/Aluminium/LDPE, in a carton.
Package sizes: 20, 30, 40, or 60 ampoules in a carton.
Not all package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Teva Pharmaceuticals Polska Sp. z o.o.
ul. Emilii Plater 53
00-113 Warsaw
Manufacturer/Importer
Merckle GmbH
Ludwig-Merckle-Str. 3
89143 Blaubeuren
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium
Budesonide Teva 0.125 mg/ml, nebuliser suspension
Bulgaria
Infaresp 0.5 mg/2 ml nebuliser suspension
Denmark
Budesonide Teva Pharma
Estonia
Budesonide Teva
Finland
Budesonide Teva 0.5 mg/ml nebuliser suspension
Ireland
Budesonide Teva Pharma 0.5 mg/2 ml and 1 mg/2 ml Nebuliser Suspension
Lithuania
Budesonide Teva 0.5mg/2 ml nebuliser suspension
Netherlands
Budesonide Teva Steri-Neb 0.25 mg/2 ml, nebuliser suspension
Norway
Budesonid Teva
Poland
Nebbud
Sweden
Budesonide Teva Pharma
Date of last revision of the leaflet:May 2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterMerckle GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NebbudDosage form: Suspension, 0.125 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.25 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.5 mg/mlActive substance: budesonidePrescription required
Alternatives to Nebbud in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nebbud in Ukraine
Alternative to Nebbud in Spain
Online doctors for Nebbud
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nebbud – subject to medical assessment and local rules.