
Nasometin Control

Ask a doctor about a prescription for Nasometin Control

How to use Nasometin Control
Before using the medicine, read the leaflet carefully and familiarize yourself with the educational materials.
These materials are available at pharmacies.
Detailed and up-to-date information about this medicine is available after scanning the QR code using a smartphone (see also point 6 of the leaflet).
Leaflet attached to the packaging: patient information
Nasometin Control, 50 micrograms/dose, nasal spray, suspension
Mometasone furoate
For use only in adults
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as advised by a doctor or pharmacist.
- Keep this leaflet to be able to read it again if necessary.
- If advice or additional information is needed, consult a pharmacist.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
- If there is no improvement after 14 days or if the patient feels worse, they should contact their doctor.
Table of contents of the leaflet:
- 1. What is Nasometin Control and what is it used for
- 2. Important information before using Nasometin Control
- 3. How to use Nasometin Control
- 4. Possible side effects
- 5. How to store Nasometin Control
- 6. Contents of the packaging and other information
1. What is Nasometin Control and what is it used for
What is Nasometin Control?
Nasometin Control contains the active substance mometasone furoate, a corticosteroid. Mometasone furoate sprayed into the nose can help alleviate inflammation (swelling and irritation of the nasal mucosa).
What is Nasometin Control used for?
Nasometin Control is used to treat symptoms of seasonal allergic rhinitis (also known as hay fever) diagnosed by a doctor in adults aged 18 and over. Hay fever, which occurs several times a year, is an allergic reaction caused by inhaled pollen from trees, grasses, weeds, as well as molds and fungal spores. Nasometin Control reduces swelling and irritation of the nasal mucosa, thereby relieving sneezing, itching, and nasal congestion or runny nose caused by hay fever. If there is no improvement after 14 days or if the patient feels worse, they should contact their doctor.
2. Important information before using Nasometin Control
When not to use Nasometin Control
if the patient is allergic to mometasone furoate or any of the other ingredients of this medicine (listed in section 6). if the patient has an untreated infection of the nasal mucosa. Using Nasometin Control during such an infection (e.g., herpes) may worsen the course of the disease. The use of the nasal spray should be delayed until the infection has resolved. if the patient has recently undergone nasal surgery or nasal injury. The nasal spray should not be used until the wounds in the nose have healed.
Warnings and precautions
Before startingto use Nasometin Control, the patient should discuss it with their doctor or pharmacist if they: have or have had tuberculosis; have any other infection; are taking other corticosteroids (orally or by injection); have cystic fibrosis. Duringthe use of Nasometin Control, the patient should consult their doctor or pharmacist if: their immune system is not functioning properly (they have trouble fighting infections) and they come into contact with someone who has chickenpox or shingles. They should avoid contact with such people; they have a nasal or throat infection; they use the medicine for 3 months without consulting a doctor; they have persistent nasal or throat irritation. If the patient experiences blurred vision or other vision disturbances, they should consult their doctor. Long-term use of high doses of corticosteroids in nasal sprays may cause side effects due to the absorption of the medicine into the body. If the patient experiences itching or irritation of the eyes, the doctor may recommend using other medicines in addition to Nasometin Control.
Children and adolescents
Nasometin Control should not be used in children and adolescents under the age of 18.
Nasometin Control and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to use, including those available without a prescription. Some medicines may enhance the effect of Nasometin Control. The doctor may want to closely monitor the patient's condition when taking such medicines (including certain medicines used to treat HIV infection: ritonavir, cobicistat). If the patient is using other corticosteroids in oral or injectable form to treat allergies, the doctor may advise them to stop using them when starting to use Nasometin Control. In some patients, after stopping the use of corticosteroids in oral or injectable form, certain side effects may occur, such as joint or muscle pain, weakness, and depression. Other allergic symptoms, such as itching, tearing of the eyes, or red and itchy spots on the skin, may also occur. If the patient experiences any of these symptoms, they should consult their doctor.
Pregnancy and breastfeeding
There is limited or no data on the use of Nasometin Control in pregnant women. It is not known whether mometasone furoate passes into breast milk. If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Driving and using machines
The effect of Nasometin Control on the ability to drive and use machines is not known.
Nasometin Control contains benzalkonium chloride
Nasometin Control contains benzalkonium chloride, which may cause irritation or swelling of the nasal mucosa, especially if the medicine is used for a long time.
3. How to use Nasometin Control
This medicine should always be used exactly as described in this patient leaflet or as advised by a doctor or pharmacist. In case of doubt, the patient should consult their doctor or pharmacist.
Recommended dose for adults aged 18 and over is two sprays of the nasal spray into each nostril once daily.
Once improvement is achieved, the dose can be reduced to one spray into each nostril once daily, and in case of worsening symptoms, it can be increased again to two sprays into each nostril once daily. In some patients, Nasometin Control may provide improvement after 12 hours of administration of the first dose, although the full benefits of treatment may only be visible after two days of using the medicine.
If after a maximum of 14 days of using the medicine, there is no improvement or it is insufficient, the patient should consult their doctor.
Nasometin Control should not be used for more than 3 months without consulting a doctor.
Children and adolescents should not use Nasometin Control. Preparing the medicine for useNasometin Control has a protective cap that protects the tip of the actuator and prevents it from getting dirty. The patient should remember to remove it before using the spray and put it back on after use.
Shake the bottle well before each use.
Do not puncture the actuator (the hole in the tip of the actuator) with a needle or any other sharp object.
Before first use: Before first using the spray, the patient should check its operation by pressing the bottle 10 times until a fine mist is obtained.
- 1. Shake the bottle well.
- 2. Remove the plastic protective cap.
- 3. Place the index and middle fingers on either side of the actuator and the thumb under the bottle (Figure 1). Do notpuncture the actuator.
- 4. With the actuator pointed away from them, press the actuator with their fingers to spray the medicine 10 times until a fine mist is obtained (Figure 1).
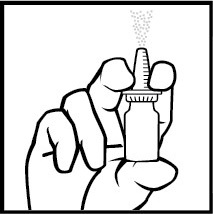
Figure 1
If the medicine has not been used for 14 days or longer, the patient should check its operation by pressing the actuator twice until a fine mist is obtained. How to use the nasal spray
- 1. Shake the bottle well and remove the protective cap (Figure 2).
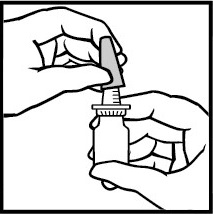
Figure 2
- 2. Gently blow their nose to clear the nasal passages.
- 3. Close one nostril with their index finger. Tilt their head slightly forward. Place the actuator between their index and middle fingers, supporting the bottle with their thumb from below. Hold the bottle upright and insert the tip of the actuator into the other nostril. It is important not to apply the medicine directly to the nasal septum, which separates the nostrils (Figure 3).
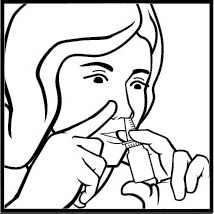
Figure 3
- 4. Press the actuator with their fingers ONCEto spray a fine mist into their nose and start a gentle and slow inhalation through their nose (Figure 4).
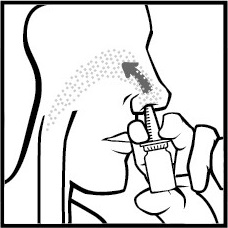
Figure 4
WARNING! Keeping the bottle upright is very important, as improper spraying may damage the nasal septum (Figure 5).
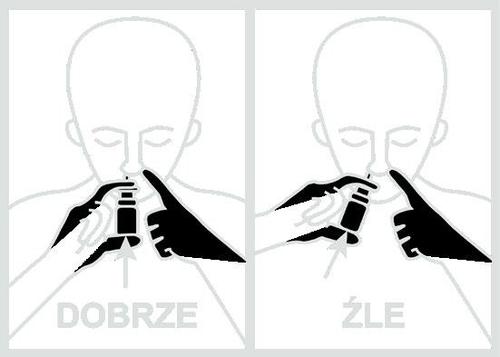
Figure 5
- 5. Exhale through their mouth. Then repeat the actions described in point 4 to administer a second dose of the spray to the same nostril (if such dosing is recommended).
- 6. Remove the actuator from the nostril and exhale through their mouth. Repeat the actions described in points 3 to 6 to administer the spray to the other nostril.
After using the spray, the patient should carefully wipe the actuator with a clean tissue or cloth and put the protective cap back on. Cleaning It is essential to regularly clean the actuator to ensure it works properly. The patient should remove the cap and gently pull off the actuator. Wash the actuator and cap in warm water, then rinse under running water.
Do not attempt to unblock the actuator by inserting a needle or any other sharp object, as this may damage the actuator and cause incorrect dosing.
Leave the cap and actuator in a warm place to dry. Put the actuator back on the bottle, then put the cap on. After cleaning, the patient should check the operation of the spray by pressing the actuator twice.
Using a higher dose of Nasometin Control than recommended
This medicine should always be used exactly as described in the patient leaflet or as advised by a doctor or pharmacist. Using a lower or higher dose of the medicine may worsen the symptoms of the disease. The patient should consult their doctor if they have accidentally used a higher dose of the medicine than recommended. If corticosteroids are used for a long time or in high doses, they may rarely have an adverse effect on the functioning of certain hormones.
Missing a dose of Nasometin Control
If the patient forgets to use the nasal spray at the usual time, they should use it as soon as they remember, and then continue treatment as recommended. They should not use a double dose to make up for the missed dose. If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. After using this medicine, immediate allergic reactions (hypersensitivity) may occur, including severe ones. If the patient experiences any of the following symptoms, they should stop using Nasometin Control and seek medical help immediately: swelling of the face, tongue, or throat difficulty swallowing hives wheezing or difficulty breathing. Long-term use of high doses of corticosteroids in nasal sprays may cause side effects due to the absorption of the medicine into the body. Other side effects Common side effects (may occur in less than 1 in 10 people): headache sneezing nasal bleeding nasal pain ulceration of the nasal mucosa respiratory tract infections. Side effects with unknown frequency (frequency cannot be estimated from the available data): increased pressure in the eye (glaucoma) and/or cataract causing vision disturbances blurred vision damage to the nasal septum changes in taste and smell breathing difficulties and/or wheezing.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of this medicine.
5. How to store Nasometin Control
Keep the medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the label and carton after EXP. The expiry date refers to the last day of the month. Do not freeze. Before using the first dose, shake the bottle well and spray the medicine 10 times (until a uniform spray is obtained). If the pump has not been used for 14 days or longer, the patient should check its operation before the next use by spraying the medicine twice until a uniform spray is obtained. The shelf life after opening the bottle is 2 months. Do not remove the pump or actuator. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines they no longer use. This will help protect the environment.
6. Contents of the packaging and other information
What Nasometin Control contains
- The active substance of the medicine is mometasone furoate. Each spray contains 50 micrograms of mometasone furoate (as mometasone furoate monohydrate). The total mass of the suspension in one spray is 100 mg.
- The other ingredients are: microcrystalline cellulose, sodium carmellose, glycerol, citric acid monohydrate, sodium citrate, polysorbate 80, benzalkonium chloride, water for injections.
What Nasometin Control looks like and what the pack contains
Nasometin Control is a white, homogeneous suspension in a nasal spray, packaged in white bottles with a spray pump and a blue protective cap. 1 bottle contains 10 g of nasal spray suspension (equivalent to 60 metered doses). 1 bottle contains 17 g of nasal spray suspension (equivalent to 120 metered doses). 1 bottle contains 18 g of nasal spray suspension (equivalent to 140 metered doses).
Marketing authorization holder and manufacturer
Marketing authorization holder Sandoz GmbH Biochemiestrasse 10 6250 Kundl, Austria Manufacturer Lek Pharmaceuticals d.d. Verovškova 57 1526 Ljubljana, Slovenia
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany Mometasone furoate - 1 A Pharma Heuschnupfenspray 50 Mikrogramm/Sprühstoß Nasenspray, Suspension Bulgaria MomaNose 50 micrograms/actuation, nasal spray, suspension Czech Republic Momanose Denmark Mommox Estonia Nasometin Croatia Momanose 50 mikrograma sprej za nos, suspenzija Hungary Momepax Control 50 mikrogramm/adag szuszpenziós orrspray Ireland Rhinex Relief 50 micrograms/actuation nasal spray, suspension Italy Mometasone Sandoz BV Lithuania Nasometin 50 mikrogramų/dozėje nosies purškalas (suspensija) Latvia Nasometin 50 mikrogrami/izsmidzinājumā deguna aresols, suspensija Poland Nasometin Control Romania Rinamet 50 micrograme/doză, spray nazal, suspensie Slovenia Mommox RINO 50 mikrogramov/vpih pršilo za nos, suspenzija Slovakia Nasometin 0,05 mg/dávka
For more information about this medicine, the patient should contact their local representative of the marketing authorization holder:
Sandoz Polska Sp. z o.o. ul. Domaniewska 50 C 02-672 Warsaw phone: 22 209 70 00 Detailed and up-to-date information about this medicine is available after scanning the QR code using a smartphone, which can be found in the leaflet below and on the outer packaging. The same information is also available on the website: https://www.sandoz.pl/sites/www.sandoz.pl/files/Nasometin%20Control_0.pdf QR Date of last revision of the leaflet:12/2023 (Logo of the marketing authorization holder)
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterLek Pharmaceuticals d.d.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Nasometin ControlDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FARMEA US Pharmacia Sp. z o.o.Prescription not requiredDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FarmeaPrescription requiredDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: Adamed Pharma S.A. FarmeaPrescription not required
Alternatives to Nasometin Control in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nasometin Control in Spain
Alternative to Nasometin Control in Ukraine
Online doctors for Nasometin Control
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nasometin Control – subject to medical assessment and local rules.







