
Nasometin
Ask a doctor about a prescription for Nasometin

How to use Nasometin
Leaflet attached to the packaging: patient information
Nasometin, 50 micrograms/dose, nasal spray, suspension
Mometasone furoate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for a specific person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Nasometin and what is it used for
- 2. Important information before using Nasometin
- 3. How to use Nasometin
- 4. Possible side effects
- 5. How to store Nasometin
- 6. Contents of the packaging and other information
1. What is Nasometin and what is it used for
What is Nasometin?
Nasometin nasal spray contains mometasone furoate, which belongs to a group of medicines called corticosteroids. Mometasone furoate sprayed into the nose can help relieve inflammation (swelling and irritation of the nasal mucosa), sneezing, itching, and a feeling of nasal congestion or runny nose.
What is Nasometin used for?
Hay fever and perennial allergic rhinitis
Nasometin is used to treat the symptoms of hay fever (also known as seasonal allergic rhinitis) and perennial allergic rhinitis in adults and children aged 3 years and older.
Hay fever, which occurs several times a year, is an allergic reaction caused by inhaled pollen from trees, grasses, weeds, as well as molds and fungal spores. Perennial allergic rhinitis occurs throughout the year, and symptoms can be caused by hypersensitivity to various factors, including house dust mites, animal dander or skin, feathers, and some foods.
Nasometin reduces swelling and irritation of the nasal mucosa, thereby relieving sneezing, itching, and nasal congestion or runny nose caused by hay fever or perennial allergic rhinitis.
Nasal polyps
Nasometin is used to treat nasal polyps in adults aged 18 years and older.
Nasal polyps are small growths inside the nose, usually in both nostrils. Nasometin reduces inflammation, which causes the polyps to shrink gradually and relieve the feeling of nasal congestion, making it easier to breathe through the nose.
2. Important information before using Nasometin
When not to use Nasometin
Warnings and precautions
Before starting to use Nasometin, the patient should discuss it with their doctor or pharmacist if they: Long-term use of high doses of corticosteroids in nasal sprays may cause certain side effects in children, such as slowed growth rate. The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those available without a prescription. There is limited or no data on the use of Nasometin in pregnant women. It is not known whether mometasone furoate passes into breast milk. The effect of Nasometin on the ability to drive and use machines is not known. This medicine contains benzalkonium chloride, which may cause irritation or swelling inside the nose, especially if used for a long time. This medicine should always be used as directed by the doctor. If in doubt, the patient should consult their doctor or pharmacist. The nasal spray should not be used in larger doses, more frequently, or for longer than prescribed by the doctor. The recommended dose is 2 sprays into each nostril once daily. The recommended dose is 1 spray into each nostril once daily. The recommended initial dose is 2 sprays into each nostril once daily. Before using the spray for the first time, the patient should check that it is working by pressing the bottle 10 times until a fine mist is produced. If the medicine has not been used for 14 days or longer, the patient should check that it is working by pressing the bottle 2 times until a fine mist is produced. Figure 3 After using the spray, the patient should carefully wipe the tip of the actuator with a clean tissue or cloth and replace the cap. Leave the cap and actuator in a warm place to dry. The patient should contact their doctor if they have accidentally used a higher dose of the medicine than recommended. If the patient forgets to use the nasal spray at the usual time, they should use it as soon as they remember, and then continue with the treatment as recommended. In some patients, Nasometin may provide improvement within 12 hours of the first dose, although the full benefits of treatment may only be apparent after 2 days of using the medicine. Like all medicines, this medicine can cause side effects, although not everybody gets them. If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Keep the medicine out of the sight and reach of children. This medicine is a white, homogeneous suspension, packaged in white plastic bottles with a pump and actuator, and placed in a cardboard box. Marketing authorization holder Sandoz Polska Sp. z o.o.Children
Nasometin and other medicines
Pregnancy and breastfeeding
Driving and using machines
Nasometin contains benzalkonium chloride
3. How to use Nasometin
Treatment of hay fever and perennial allergic rhinitis
Use in adults and children over 12 years of age
Use in children from 3 to 11 years of age
Nasal polyps
Use in adults over 18 years of age
Nasometin has a cap that protects the tip of the actuator and prevents it from getting dirty. The patient should remember to remove it before using the spray and put it back on after use.Before each use, shake the bottle.
Do not puncture the actuator (the hole in the tip of the actuator) with a needle or other sharp object.
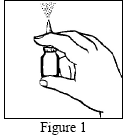
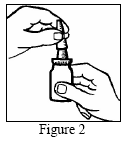
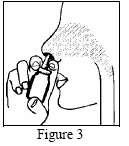
Do not attempt to unblock the actuator by inserting a needle or other sharp object into the hole, as this may damage the actuator and cause the medicine to be administered incorrectly.
Using a higher dose of Nasometin than recommended
Missing a dose of Nasometin
Stopping the use of Nasometin
4. Possible side effects
Reporting side effects
5. How to store Nasometin
6. Contents of the packaging and other information
What Nasometin contains
What Nasometin looks like and contents of the pack
Marketing authorization holder and manufacturer
For more information about this medicine and its names in the Member States of the European Economic Area, please contact:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLek Pharmaceuticals d.d.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NasometinDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FARMEA US Pharmacia Sp. z o.o.Prescription not requiredDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FarmeaPrescription requiredDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: Adamed Pharma S.A. FarmeaPrescription not required
Alternatives to Nasometin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nasometin in Іспанія
Alternative to Nasometin in Україна
Online doctors for Nasometin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nasometin – subject to medical assessment and local rules.








