
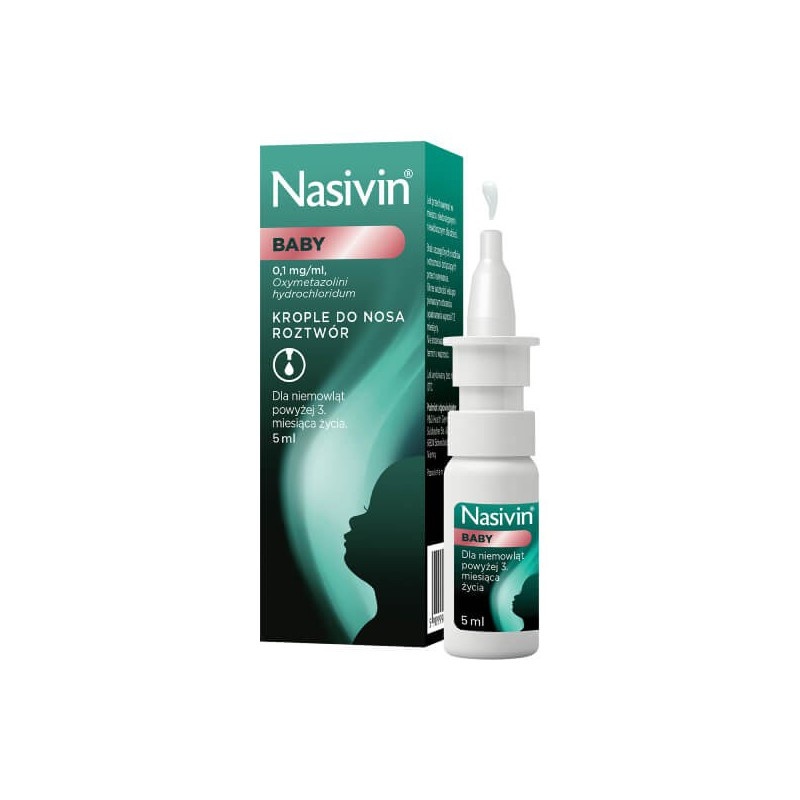
Nasivin Vicks Babi

Ask a doctor about a prescription for Nasivin Vicks Babi

How to use Nasivin Vicks Babi
Package Leaflet: Information for the Patient
Nasivin Vicks Baby
0.1 mg/ml, nasal drops, solution
Oxymetazoline hydrochloride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient or as directed by a doctor or pharmacist.
- This leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, the doctor or pharmacist should be informed. See section 4.
- If there is no improvement after 5 to 7 days or the patient feels worse, a doctor should be consulted.
Table of Contents of the Leaflet:
- 1. What is Nasivin Vicks Baby and what is it used for
- 2. Important information before using Nasivin Vicks Baby
- 3. How to use Nasivin Vicks Baby
- 4. Possible side effects
- 5. How to store Nasivin Vicks Baby
- 6. Contents of the pack and other information
1. WHAT IS NASIVIN VICKS BABY AND WHAT IS IT USED FOR
Nasivin Vicks Baby contains the active substance oxymetazoline hydrochloride, which narrows blood vessels. Administration of Nasivin Vicks Baby reduces swelling of the inflamed mucous membrane and inhibits excessive secretion, making it easier to breathe through the nose. The reduction of nasal mucosal congestion also leads to the opening and dilation of the paranasal sinuses and unblocks the Eustachian tubes. This facilitates the removal of secretions and the healing of bacterial infections.
Nasivin Vicks Baby does not contain preservatives.
Nasivin Vicks Baby is used for swelling of the mucous membranes occurring in acute rhinitis, vasomotor rhinitis, allergic rhinitis, sinusitis, otitis media, and Eustachian tube inflammation.
2. IMPORTANT INFORMATION BEFORE USING NASIVIN VICKS BABY
When not to use Nasivin Vicks Baby:
- if the patient has dry rhinitis (rhinitis with crust formation);
- if the patient is allergic (hypersensitive) to oxymetazoline hydrochloride or any of the other ingredients of Nasivin Vicks Baby (listed in section 6).
Nasivin Vicks Baby may be used with special caution and only after careful consideration by a doctor of the benefit-risk ratio in the case of:
- treatment with monoamine oxidase inhibitors (MAOIs) and other drugs that may increase blood pressure;
- increased intraocular pressure, especially in the case of angle-closure glaucoma;
- severe heart and blood vessel diseases (e.g., coronary heart disease, hypertension);
- phaeochromocytoma;
- metabolic disorders (e.g., hyperthyroidism, diabetes).
When to exercise special caution when using Nasivin Vicks Baby
The effectiveness of nasal decongestants may decrease (tachyphylaxis) due to their prolonged use or overdose. This can lead to the use of increasingly larger doses or more frequent use of the medicine, which in turn can result in permanent use of the medicine. In the case of prolonged use or abuse of the medicine, its use should be stopped immediately.
Permanent use of the medicine can lead to a breathing difficulty caused by reactive nasal mucosal congestion (rebound effect) and chronic, drug-induced rhinitis (rhinitis medicamentosa), as well as atrophy of the nasal mucosa or dry rhinitis (rhinitis sicca). The rebound effect and tachyphylaxis should resolve after discontinuation of the medicine.
Similarly, abuse of locally applied nasal drugs can lead to atrophy of the nasal mucosa and reactive congestion with drug-induced rhinitis (rhinitis medicamentosa).
Use for longer than recommended or overdose of the medicine should be avoided.
Nasivin Vicks Baby and other medicines
Using medicines containing oxymetazoline, such as Nasivin Vicks Baby, at the same time as certain medicines used in depression, such as MAOIs of the tranylcypromine type and tricyclic antidepressants, may lead to an increase in blood pressure.
Pregnancy and breastfeeding
Nasivin Vicks Baby is intended for use in infants over 3 months of age up to 1 year of age.
Before using any medicine in pregnant or breastfeeding women, a doctor or pharmacist should be consulted.
Cautious use of the medicine during pregnancy and breastfeeding is recommended. The recommended dose should not be exceeded.
Driving and using machines
Nasivin Vicks Baby is intended for use in infants over 3 months of age up to 1 year of age. In the case of use by adults, it should be taken into account that during the use of oxymetazoline, especially for a long period or in doses larger than recommended, systemic effects on the cardiovascular and central nervous systems may occur. In such cases, the ability to drive vehicles and operate machinery may be impaired.
3. HOW TO USE NASIVIN VICKS BABY
Nasivin Vicks Baby should be used as directed by a doctor or as described in this leaflet. In case of doubts, a doctor should be consulted.
Nasivin Vicks Baby is intended exclusively for intranasal administration (also in Eustachian tube inflammation and otitis media).
The packaging of the medicine should be used by only one person.
Infants over 3 months of age up to 1 year of age:
1 to 2 drops of Nasivin Vicks Baby should be instilled into each nostril 2 to 3 times a day.
The medicine should not be used for longer than 5 to 7 days.
Method of administration
The method of administration of the medicine is shown in the diagram below. Before the first use, the pump should be pressed several times to obtain a full dose.
Since the bottle with the dosing pump must be held upside down during administration, the administration of Nasivin Vicks Baby is possible only when the child's head is tilted back (Fig. 1).
Fig.1
Use of a higher than recommended dose of Nasivin Vicks Baby
In the case of overdose or accidental ingestion of the medicine, the following symptoms may occur: dilated pupils, nausea, vomiting, cyanosis (blue discoloration of the skin and mucous membranes), fever, muscle spasms, tachycardia (rapid heart rate), arrhythmias, circulatory collapse (sudden deterioration of blood circulation), cardiac arrest, increased blood pressure, pulmonary edema (fluid accumulation in the lungs, manifested by shortness of breath), respiratory disorders, and mental disorders.
Additionally, there may be: central nervous system depression, drowsiness, decreased body temperature, bradycardia (slow heart rate), sudden decrease in blood pressure as in shock, apnea, and coma.
In the case of use of a higher than recommended dose of the medicine, a doctor should be consulted immediately.
Missed dose of Nasivin Vicks Baby
A double dose should not be used to make up for a missed dose.
In case of any further doubts related to the use of the medicine, a doctor or pharmacist should be consulted.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Nasivin Vicks Baby can cause side effects, although not everybody gets them.
Frequent side effects (in less than 1 in 10, but more than 1 in 100 patients using the medicine):
burning or dryness of the nasal mucosa, sneezing, especially in sensitive patients.
Uncommon side effects (in less than 1 in 100, but more than 1 in 1,000 patients using the medicine):
systemic effects, such as palpitations, rapid heart rate, and increased blood pressure, occurring after local (intranasal) use of the medicine.
Rare side effects (in less than 1 in 1,000, but more than 1 in 10,000 patients using the medicine):
headache, insomnia, or fatigue.
In rare cases, after the effect of the medicine has worn off, there may be an exacerbation of nasal mucosal congestion (so-called reactive congestion).
Prolonged or frequent use, as well as use of higher than recommended doses of Nasivin Vicks Baby, may lead to reactive congestion with drug-induced rhinitis (rhinitis medicamentosa). Such an effect may occur as early as 5-7 days after treatment and, in the case of continued use of the medicine, may lead to permanent damage to the nasal mucosa.
Long-term use or abuse of oxymetazoline may also lead to a decrease in the effectiveness of the medicine (tachyphylaxis) (frequency not known - cannot be estimated from the available data).
If any of the side effects worsen or any side effects not listed in the leaflet occur, a doctor or pharmacist should be informed.
Reporting of side effects
If any side effects occur, including any possible side effects not listed in the leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw, phone: +48 22 49-21-301, fax: +48 22 49-21-309,
e-mail: [email protected]. By reporting side effects, more information can be collected on the safety of the use of the medicine.
Side effects can also be reported to the marketing authorization holder.
5. HOW TO STORE NASIVIN VICKS BABY
The medicine should be stored in a place inaccessible and invisible to children.
No special precautions for storage are required.
The shelf life of Nasivin Vicks Baby after the first opening of the packaging is 12 months.
Nasivin Vicks Baby should not be used after the expiry date stated on the packaging.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Nasivin Vicks Baby contains
- The active substance of the medicine is oxymetazoline hydrochloride. 1 ml of the solution contains 0.1 mg of oxymetazoline hydrochloride. 1 drop of the solution contains 2.8 micrograms of oxymetazoline hydrochloride.
- The other ingredients of the medicine are: citric acid monohydrate, sodium citrate, glycerol 85%, purified water.
What Nasivin Vicks Baby looks like and what the pack contains
The packaging contains a polyethylene bottle with a dosing pump in a 3K system containing 5 ml of the solution, in a cardboard box.
Marketing authorization holder:Wick Pharma Zweigniederlassung der Procter & Gamble GmbH
Sulzbacher Str. 40
65824 Schwalbach am Taunus
Germany
Manufacturer:
Famar Health Care Services Madrid, S.A.U.
Avda. Leganés, 62
Alcorcón 28923 (Madrid)
Spain
Sofarimex - Indústria Química e Farmacêutica, S.A.
Av. das Indústrias - Alto do Colaride
- 2735 - 213 Cacém Portugal
Date of the last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterFamar Health Care Services Madrid SAU Sofarimex Industria Quimica e Farmaceutica S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Nasivin Vicks BabiDosage form: Aerosol, 0.25 mg/mlActive substance: oxymetazolineManufacturer: Lomapharm GmbHPrescription not requiredDosage form: Aerosol, 0.5 mg/mlActive substance: oxymetazolineManufacturer: Lomapharm GmbHPrescription not requiredDosage form: Aerosol, 0.5 mg/ml (0.05%)Active substance: oxymetazolinePrescription not required
Alternatives to Nasivin Vicks Babi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nasivin Vicks Babi in Ukraine
Alternative to Nasivin Vicks Babi in Spain
Online doctors for Nasivin Vicks Babi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nasivin Vicks Babi – subject to medical assessment and local rules.








