
Acatar Care

Ask a doctor about a prescription for Acatar Care

How to use Acatar Care
Leaflet attached to the packaging: patient information
Acatar Care
0.5 mg/mL, nasal spray, solution
Oxymetazoline hydrochloride
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as advised by your doctor or pharmacist.
- You should keep this leaflet, so you can read it again if you need to.
- If you need advice or additional information, you should consult your pharmacist.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. See section 4.
- If after 5 to 7 days there is no improvement or you feel worse, you should contact your doctor.
Table of contents of the leaflet
- 1. What is Acatar Care and what is it used for
- 2. Important information before using Acatar Care
- 3. How to use Acatar Care
- 4. Possible side effects
- 5. How to store Acatar Care
- 6. Contents of the packaging and other information
1. What is Acatar Care and what is it used for
Acatar Care contains the active substance oxymetazoline hydrochloride, which narrows blood vessels. Administration of Acatar Care reduces swelling of the inflamed mucous membrane and inhibits excessive secretion, making it easier to breathe through the nose. The reduction of nasal mucosal congestion also leads to the opening and dilation of the paranasal sinuses and unclogs the Eustachian tubes. This facilitates the removal of secretions and the healing of bacterial infections.
Acatar Care, compared to a physiological saline solution, shortens the average duration of acute rhinitis (stuffy nose, runny nose, sneezing, malaise) from 6 to 4 days.
Acatar Care is used in swelling of the mucous membranes occurring in acute rhinitis, vasomotor rhinitis, allergic rhinitis, sinusitis, Eustachian tube inflammation, and middle ear inflammation.
The effect of oxymetazoline lasts up to 12 hours. In studies, the onset of action of the medicine was observed on average after 25 seconds.
Acatar Care contains the auxiliary substance sodium hyaluronate, which protects and moisturizes the nasal mucosa.
Acatar Care does not contain preservatives.
2. Important information before using Acatar Care
1/6
When not to use Acatar Care
- treatment with monoamine oxidase inhibitors (MAOIs) and other medicines that may increase blood pressure;
- increased intraocular pressure, especially in the case of angle-closure glaucoma;
- severe heart and blood vessel diseases (e.g., coronary heart disease, hypertension);
- chromaffin cell tumor of the adrenal gland;
- metabolic disorders (e.g., hyperthyroidism, diabetes).
Warnings and precautions
The effectiveness of decongestant nasal sprays may decrease (tachyphylaxis) due to their prolonged use or overdose. This can lead to the use of increasingly larger doses or more frequent use of the medicine, which in turn can result in permanent use of the medicine. In the case of prolonged use or abuse of the medicine, its use should be stopped immediately.
Permanent use of the medicine can lead to a breathing difficulty caused by reactive nasal mucosal congestion (rebound effect) and chronic, drug-induced rhinitis (rhinitis medicamentosa), as well as atrophy of the mucous membrane or dry rhinitis (rhinitis sicca). The rebound effect and tachyphylaxis should resolve after discontinuation of the medicine.
Similarly, abuse of locally applied nasal sprays can lead to atrophy of the mucous membrane and reactive congestion with drug-induced rhinitis (rhinitis medicamentosa).
Use of the medicine for a longer period than recommended or in excessive doses should be avoided.
Children and adolescents
Acatar Care is intended for use in children and adolescents over 6 years of age.
Acatar Care and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Using medicines containing oxymetazoline, such as Acatar Care, at the same time as certain antidepressant medicines, such as MAOIs of the tranylcypromine type or tricyclic or tetracyclic antidepressants, may lead to increased blood pressure.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Cautious use of the medicine is recommended during pregnancy or breastfeeding. The recommended dose should not be exceeded.
Driving and operating machinery
During use of oxymetazoline for a long period or in doses larger than recommended, systemic effects on the circulatory system and central nervous system may occur. In such cases, the ability to drive vehicles or operate machinery may be impaired.
3. How to use Acatar Care
This medicine should always be used exactly as described in the patient leaflet or as advised by your doctor or pharmacist. If you have any doubts, you should consult your doctor or pharmacist.
Acatar Care is intended for nasal use only (also in Eustachian tube inflammation and middle ear inflammation).
The packaging of the medicine should only be used by one person.
Acatar Care can only be used in adults and children over 6 years of age.
The medicine should not be used in infants and children under 6 years of age.
Adults and children over 6 years of age: 1 dose of Acatar Care spray into each nostril 2 to 3 times a day.
The medicine should not be used for more than 5 to 7 days.
Instructions for using the medicine
Before the first use, you should press the pump several times to obtain a full dose.
Holding your head in an upright position (not tilting it back), you should place the tip of the applicator in the nostril, without completely blocking its lumen.
Direct the tip of the dispenser away from the nasal septum (the wall between the nostrils), i.e., in the right nostril towards the right eye, and in the left nostril towards the left eye.
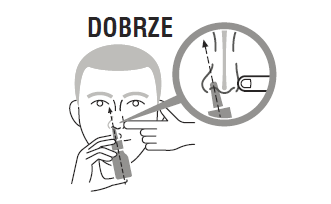
Then, quickly and energetically press the dispenser, while performing a gentle inhalation through the nose.
Do not spray the aerosol directly onto the nasal septum, as this may cause damage to the nasal mucosa.
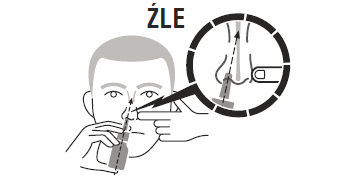
After use, you should clean the dispenser. Using the dispenser by more than one person may contribute to the spread of infection.
Using a higher dose of Acatar Care than recommended
3/6
In case of overdose or accidental ingestion of the medicine, the following symptoms may occur: dilated pupils, nausea, vomiting, cyanosis (blue discoloration of the skin and mucous membranes), fever, muscle cramps, tachycardia (accelerated heart rate), arrhythmia, circulatory collapse (sudden deterioration of blood circulation), cardiac arrest, increased blood pressure, pulmonary edema (fluid accumulation in the lungs, manifested by shortness of breath), respiratory disorders, mental disorders.
Additionally, there may be inhibition of the central nervous system, drowsiness, decreased body temperature, bradycardia (slow heart rate), sudden drop in blood pressure as in shock, apnea, and coma.
In case of using a higher dose of Acatar Care than recommended, you should immediately contact your doctor.
Missing a dose of Acatar Care
You should not use a double dose to make up for a missed dose.
If you have any doubts about using this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The frequency of side effects is classified as follows:
Common(may occur in up to 1 in 10 people): burning or dryness of the nasal mucosa, sneezing, especially in sensitive patients.
Uncommon(may occur in less than 1 in 100 people): systemic effects, such as palpitations, accelerated heart rate, and increased blood pressure, occurring after local (nasal) use of the medicine.
Rare(may occur in less than 1 in 1,000 people): headache, insomnia, or fatigue.
In rare cases, after the effect of the medicine has worn off, there may be an exacerbation of nasal mucosal congestion (so-called reactive congestion).
Prolonged or frequent use, as well as use of higher doses of Acatar Care than recommended, may lead to reactive congestion with drug-induced rhinitis.
Such an effect may occur after 5-7 days of treatment and, in the case of continued use of the medicine, may lead to permanent damage to the nasal mucosa.
Long-term use or abuse of oxymetazoline may also lead to a decrease in the effectiveness of the medicine (tachyphylaxis) (unknown frequency of occurrence - frequency cannot be determined based on available data).
If any of the side effects worsen or if you experience any side effects not listed in the leaflet, you should inform your doctor or pharmacist.
Reporting side effects
If you experience any side effects, including those not listed in the leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
4/6
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Acatar Care
The medicine should be stored out of sight and reach of children.
There are no special recommendations for storing the medicinal product.
The shelf life of Acatar Care after opening the packaging is 12 months.
Acatar Care should not be used after the expiry date stated on the packaging.
The expiry date refers to the last day of the given month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Acatar Care contains
The active substance of the medicine is oxymetazoline hydrochloride. 1 mL of the solution contains 0.5 mg of oxymetazoline hydrochloride.
The other ingredients are: glycerol 85%, sodium citrate, citric acid monohydrate, sodium hyaluronate, hydrochloric acid 25% (for pH adjustment), sodium hydroxide (for pH adjustment), water for injections.
1 dose of the spray with a volume of 45 microliters contains 22.5 micrograms of oxymetazoline hydrochloride.
What Acatar Care looks like and what the packaging contains
Clear, colorless solution.
HDPE bottle containing 15 mL of solution, with a dosing pump made of HDPE, LDPE/PP, PTFE/PET (in the APF system), and a cap made of LDPE, in a cardboard box.
Marketing authorization holder
US Pharmacia Sp. z o.o.
ul. Ziębicka 40
50-507 Wrocław
Manufacturer
Lomapharm GmbH
Langes Feld 5
Emmerthal, Niedersachsen, 31860,
Germany
For more detailed information, please contact:
USP Zdrowie Sp. z o.o.
ul. Poleczki 35
02-822 Warszawa
5/6
phone: +48 (22) 543 60 00
Date of last update of the leaflet:March 2022
6/6
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterLomapharm GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Acatar CareDosage form: Aerosol, 0.25 mg/mlActive substance: oxymetazolineManufacturer: Lomapharm GmbHPrescription not requiredDosage form: Aerosol, 0.5 mg/ml (0.05%)Active substance: oxymetazolinePrescription not requiredDosage form: Aerosol, 0.5 mg/mlActive substance: oxymetazolineManufacturer: Schering-Plough Labo N.V.Prescription not required
Alternatives to Acatar Care in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Acatar Care in Ukraine
Alternative to Acatar Care in Spain
Online doctors for Acatar Care
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Acatar Care – subject to medical assessment and local rules.








