
Naloxonum hidrohloricum Vzf

Ask a doctor about a prescription for Naloxonum hidrohloricum Vzf

How to use Naloxonum hidrohloricum Vzf
Leaflet attached to the packaging: patient information
NALOXONUM HYDROCHLORICUM WZF, 400 micrograms/ml, solution for injection
Naloxone hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist, or nurse.
- This medicine has been prescribed to a specific person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Naloxonum hydrochloricum WZF and what is it used for
- 2. Important information before using Naloxonum hydrochloricum WZF
- 3. How to use Naloxonum hydrochloricum WZF
- 4. Possible side effects
- 5. How to store Naloxonum hydrochloricum WZF
- 6. Contents of the packaging and other information
1. What is Naloxonum hydrochloricum WZF and what is it used for
Naloxonum hydrochloricum WZF contains the active substance naloxone and belongs to a group of medicines called opioid antagonists.
Naloxonum hydrochloricum WZF reverses (blocks) the unwanted effects of strong painkillers - opioids (e.g. morphine), such as breathing difficulties and drowsiness.
Naloxonum hydrochloricum WZF is used:
- in case of overdose of strong painkillers (opioids) and severe breathing difficulties;
- to reverse the effect of opioids used during surgery;
- to treat breathing difficulties in newborns whose mothers received opioids during delivery;
- to determine if the cause of poisoning is opioids.
Naloxonum hydrochloricum WZF is always administered by medical personnel.
2. Important information before using Naloxonum hydrochloricum WZF
When not to use Naloxonum hydrochloricum WZF:
- if the patient is allergic to naloxone or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Naloxonum hydrochloricum WZF, the patient should discuss it with their doctor or pharmacist.
The doctor will exercise special caution when using Naloxonum hydrochloricum WZF and will take appropriate action:
- if the patient has taken an overdose of opioids or is addicted to opioids, acute withdrawal syndrome may develop, with symptoms such as high blood pressure, rapid heartbeat, severe breathing difficulties, or cardiac arrest, which can sometimes be fatal;
- if withdrawal symptoms occur, such as nausea, vomiting, diarrhea, weakness, rapid heartbeat, increased blood pressure, fever, runny nose, sneezing, piloerection (so-called "goosebumps"), sweating, yawning, nervousness, anxiety, irritability, tremors, abdominal cramps, pain in various parts of the body; in newborns, seizures, excessive crying, and increased reflexes may occur;
- if Naloxonum hydrochloricum WZF is to be administered to a newborn whose mother took opioids before delivery or is addicted to opioids, as severe withdrawal symptoms may occur in the newborn;
- if the patient has heart disease or cardiovascular disease, or is taking heart medications, or medications with undesirable effects on the cardiovascular system, as these patients are at higher risk of side effects, such as irregular heartbeat, high or low blood pressure, or breathing difficulties;
- if the patient has used a strong painkiller - buprenorphine or pentazocine, as the effectiveness of Naloxonum hydrochloricum WZF is limited in this case;
- if breathing difficulties occur after using Naloxonum hydrochloricum WZF, as this may be due to the shorter duration of action of naloxone compared to opioids;
- if Naloxonum hydrochloricum WZF is administered after surgery, during which opioids were used, as symptoms such as nausea, vomiting, tremors, high blood pressure, and rapid heartbeat may occur (see section 4);
- if the patient has liver or kidney disease.
Naloxonum hydrochloricum WZF and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
The patient should inform their doctor if they are taking or have taken:
- strong painkillers (e.g. morphine, codeine, buprenorphine, pentazocine);
- sleeping pills and/or sedatives;
- medicines that may affect the heart or cardiovascular system (e.g. medicines used to treat high blood pressure, tricyclic antidepressants, calcium channel blockers, beta blockers, digoxin, clonidine, cocaine, methamphetamine). Severe high blood pressure has been reported during the administration of naloxone hydrochloride in cases of clonidine-induced coma.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
The patient should tell their doctor if they:
- are pregnant;
- think they may be pregnant;
- plan to become pregnant;
- are breastfeeding. The use of Naloxonum hydrochloricum WZF during pregnancy or breastfeeding is decided by the doctor. Naloxonum hydrochloricum WZF may cause withdrawal symptoms in newborns (see information above). Naloxone may only be used in pregnant women when, in the doctor's opinion, its use is absolutely necessary. Breastfeeding should be avoided for 24 hours after using the medicine.
Driving and using machines
The patient should not drive or operate machinery for at least 24 hours after using the medicine.
Naloxonum hydrochloricum WZF contains sodium
The medicine contains 3.36 mg of sodium (the main component of common salt) per 1 ml of solution. This corresponds to 0.17% of the maximum recommended daily intake of sodium in the diet for adults.
The medicine contains 84 mg of sodium (the main component of common salt) in the maximum daily dose of 10 mg of naloxone (25 ml of solution). This corresponds to 4.2% of the maximum recommended daily intake of sodium in the diet for adults.
The medicine can be diluted - see below "Information intended exclusively for medical professionals". When calculating the total sodium content in the prepared, diluted solution, the sodium content from the diluent solution should be taken into account. To obtain accurate information about the sodium content in the diluent solution, consult the product characteristics of the medicinal product used as the diluent solution.
3. How to use Naloxonum hydrochloricum WZF
Naloxonum hydrochloricum WZF is always administered by medical personnel.
The medicine can be administered:
- intravenously - injected directly into a vein or administered in a drip;
- intramuscularly;
- subcutaneously. The dose of the medicine is determined by the doctor. The dose used depends on the patient's weight, condition, and the type and amount of opioid used.
Using a higher dose of Naloxonum hydrochloricum WZF than recommended
- After using higher doses of naloxone, pain may recur and muscle tension may return.
- The medicine is administered by medical personnel, so it is unlikely that the patient will receive more medicine than they should.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
All medicines can cause allergic reactions, but severe allergic reactions after using this medicine are very rare. If the patient experiences wheezing, breathing difficulties, swelling of the eyelids, face, or lips, rash, or itching (especially all over the body), they should see a doctor immediately.
The following side effects have been reported:
- Very common (occurring in more than 1 in 10 patients): nausea.
Common (occurring in less than 1 in 10 patients):
- dizziness;
- headache;
- rapid heartbeat;
- low or high blood pressure;
- vomiting;
- postoperative pain.
Uncommon (occurring in less than 1 in 100 patients):
- tremors, sweating;
- heart rhythm disturbances, slow heartbeat;
- diarrhea;
- dry mouth;
- irritation of the blood vessel walls after intravenous administration of the medicine;
- local irritation, burning, and redness at the injection site;
- too rapid and deep breathing.
Rare (occurring in less than 1 in 1,000 patients):
- seizures;
- muscle tension.
Very rare (occurring in less than 1 in 10,000 patients):
- severe heart disorders (ventricular fibrillation, cardiac arrest);
- pulmonary edema (symptoms include fluid in the lungs, breathing difficulties, apnea);
- erythema multiforme (symptoms include usually extensive skin changes with blisters and rash, skin discoloration, and mucous membrane damage);
- allergic reactions (symptoms include usually hives, rhinitis, dyspnea, facial swelling), anaphylactic shock (severe allergic reaction).
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Naloxonum hydrochloricum WZF
The medicine should be stored out of sight and reach of children.
Ampoules should be stored in the outer packaging to protect them from light. Do not freeze.
Do not use this medicine after the expiry date stated on the carton and ampoule. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Naloxonum hydrochloricum WZF contains
- The active substance of the medicine is naloxone hydrochloride. Each 1 ml of solution contains 400 micrograms of naloxone hydrochloride (as naloxone hydrochloride dihydrate).
- The other ingredients are: sodium chloride, hydrochloric acid diluted (to adjust pH), water for injections.
What Naloxonum hydrochloricum WZF looks like and what the pack contains
Naloxonum hydrochloricum WZF is a colorless, clear solution for injection.
Ampoules made of colorless glass containing 1 ml of solution are packaged in a cardboard box.
The cardboard box contains 10 ampoules.
Marketing authorization holder and manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of last revision of the leaflet:
Information intended exclusively for medical professionals:
Naloxonum hydrochloricum WZF, 400 micrograms/ml, solution for injection
Naloxone hydrochloride
Method of administration of Naloxonum hydrochloricum WZF
The medicine is administered intravenously, intramuscularly, or subcutaneously, or as an intravenous infusion after prior dilution.
In the case of intravenous infusion, the solution should be diluted with sodium chloride 0.9% or glucose 5% solution as follows: 2000 micrograms (5 ml of solution containing 400 micrograms/ml of naloxone) in 500 ml of diluent. The resulting solution after dilution contains 4 micrograms of naloxone per 1 ml. The solution should be prepared immediately before administration.
Note: Naloxonum hydrochloricum WZF should not be administered in the same intravenous infusion as other medicines.
During the use of the medicine, the possibility of oxygen therapy and resuscitation treatment should be ensured, as well as access to equipment that allows for cardiopulmonary resuscitation.
Instructions for opening the ampoule
Before opening the ampoule, make sure that the entire solution is in the lower part of the ampoule.
The ampoule can be gently shaken or tapped with a finger to facilitate the flow of the solution.
A colored dot is placed on each ampoule (see Figure 1) as a mark indicating the break point below it.
- To open the ampoule, hold it vertically in both hands, with the colored dot facing each other - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
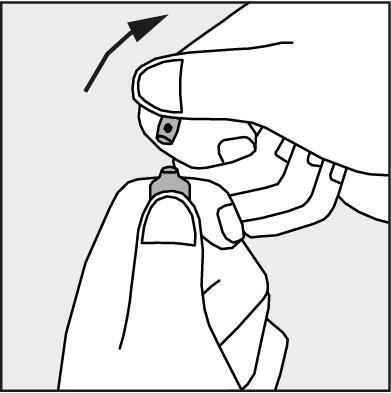
- Press in the direction of the arrow shown in Figure 3.
The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused medicine should be destroyed in accordance with applicable regulations.
Figure 1.
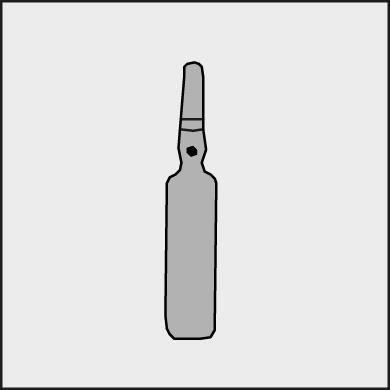
Figure 2.
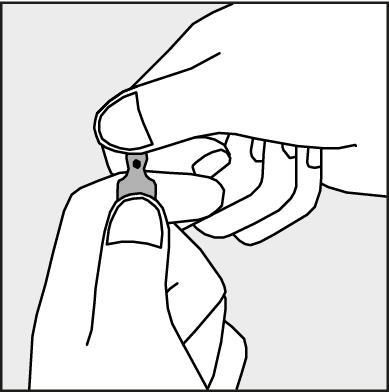
Figure 3.
Dosing of Naloxonum hydrochloricum WZF
The dose of naloxone and the route of administration depend on the patient's condition, the type and amount of opioid used.
Opioid poisoning
Adults
Usually, an initial single dose of 400 to 2000 micrograms is administered intravenously.
If necessary, the intravenous dose can be repeated every 2-3 minutes until the patient regains consciousness and regular, measured breathing. If there is no temporary improvement in respiratory function and no return of consciousness after administering 10 mg, the cause of these symptoms is probably not an opioid overdose.
The medicine can also be administered intramuscularly or subcutaneously. In life-threatening conditions, the medicine should be administered intravenously.
Children
Usually, the initial single dose administered intravenously is 10 micrograms per kilogram of body weight. If necessary, an additional dose of 100 micrograms per kilogram of body weight can be administered.
If naloxone cannot be administered intravenously, the medicine should be used intramuscularly or subcutaneously in divided doses.
Reversal of opioid-induced anesthesia
Adults
Usually, 100 micrograms to 200 micrograms are administered intravenously, i.e. 1.5-3 micrograms per kilogram of body weight.
In some cases, especially when a long-acting opioid has been used, it may be necessary to administer an additional dose of naloxone intramuscularly within 1-2 hours. The medicine can also be administered as an intravenous infusion.
Children
Intravenously, 10 micrograms per kilogram of body weight. If necessary, an additional dose of 100 micrograms per kilogram of body weight can be administered.
If naloxone cannot be administered intravenously, the medicine should be used intramuscularly or subcutaneously in divided doses. The medicine can also be administered as an intravenous infusion.
Reversal of respiratory depression in newborns caused by administration of opioid analgesics to the mother during delivery
Newborns
In case of apnea, it should be ensured that the airway is maintained before administering the medicine.
Intravenously, intramuscularly, or subcutaneously, 10 micrograms per kilogram of body weight. If necessary, the dose can be repeated after 2-3 minutes.
It is also possible to administer a single dose of naloxone intramuscularly, 200 micrograms (i.e. approximately 60 micrograms per kilogram of body weight).
Differential diagnosis of opioid poisoning
Intravenous administration of a dose of 0.5 micrograms per kilogram of body weight of naloxone allows to determine whether respiratory depression or urinary retention is not caused by an opioid. Then the dose of naloxone can be gradually increased, avoiding too high doses. High doses eliminate any effect of the opioid, including the analgesic effect, and also cause stimulation of the sympathetic nervous system and the cardiovascular system.
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Naloxonum hidrohloricum Vzf
Alternatives to Naloxonum hidrohloricum Vzf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Naloxonum hidrohloricum Vzf in Spain
Alternative to Naloxonum hidrohloricum Vzf in Ukraine
Online doctors for Naloxonum hidrohloricum Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Naloxonum hidrohloricum Vzf – subject to medical assessment and local rules.














