NYXOID 1.8 mg NASAL SPRAY SOLUTION SINGLE-DOSE CONTAINER


How to use NYXOID 1.8 mg NASAL SPRAY SOLUTION SINGLE-DOSE CONTAINER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Nyxoid 1.8 mg Nasal Spray Solution in Single-Dose Container
naloxone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Nyxoid and what is it used for
- What you need to know before you use Nyxoid
- How to use Nyxoid
- Possible side effects
- Storage of Nyxoid
- Contents of the pack and further information
1. What is Nyxoid and what is it used for
This medicine contains the active substance naloxone. Naloxone temporarily reverses the effects of opioids such as heroin, methadone, fentanyl, oxycodone, buprenorphine, and morphine.
Nyxoid is a nasal spray solution used for the emergency treatment of overdose or possible overdose of opioids in adults and adolescents aged 14 years and older. Signs of overdose include:
- breathing problems
- extreme drowsiness
- not responding to a loud noise or touch
If you are at risk of opioid overdose, you should always carry Nyxoid with you.Nyxoid only works temporarily to reverse the effects of opioids while waiting for emergency medical care. It is not a substitute for emergency medical care. Nyxoid should be used by people with the appropriate training.
Always inform your friends and family that you carry Nyxoid with you.
2. What you need to know before you use Nyxoid
Do not use Nyxoid
If you are allergic to naloxone or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Nyxoid will only be provided after you or your caregiver have received training on how to use it.
It should be administered immediately and does not replace emergency medical care.
- You should call the emergency services if an opioid overdose is suspected.
The signs and symptoms of an opioid overdose may return after administering this nasal spray. If this happens, other doses can be administered after 2 to 3 minutes, using a new Nyxoid nasal spray. After receiving this medicine, the patient should remain under close supervision until emergency help arrives.
Medical conditions to be aware of
- If you are physically dependent on opioids or have received high doses of opioids (e.g., heroin, methadone, fentanyl, oxycodone, buprenorphine, or morphine). You may experience severe withdrawal symptoms with this medicine (see section 4 of this leaflet, under “Medical conditions to be aware of”).
- If you take opioids to control pain. The pain may increase when you receive Nyxoid.
- If you use buprenorphine. Nyxoid may not completely reverse the breathing problems.
Tell your doctorif you have damage inside your nose, as this could affect the functioning of Nyxoid.
Children and adolescents
Nyxoid should not be used in children or adolescents under 14 years of age.
Administration of Nyxoid to mothers near childbirth
Tell your doctor or midwifeif you have used Nyxoidnear childbirthor during it.
Your baby may experience sudden opioid withdrawal syndrome, which could be life-threatening if not treated.
During the 24 hours following the birth of your baby, be aware of the following symptoms in your baby:
- seizures (fits)
- more crying than usual
- increased reflexes
Other medicines and Nyxoid
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. If you are given Nyxoid while pregnant or breastfeeding, your baby should be kept under close supervision.
Driving and using machines
After taking this medicine, do not drive, use machines, or engage in other activities that require physical or mental effort for at least 24 hours, as the effect of the opioids may return.
Nyxoid contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially “sodium-free”.
3. How to use Nyxoid
Follow the instructions for administration of this medicine exactly as told by your doctor, pharmacist, or nurse. If you are unsure, ask your doctor, pharmacist, or nurse again.
You will be provided with training on how to use Nyxoid before it is supplied. The following is a step-by-step guide.
Instructions for administering Nyxoid nasal spray solution
- Check for symptoms and response.
- Check for response to see if the person is conscious.You can shout their name, gently shake their shoulders, speak loudly in their ear, rub their sternum, pinch their ear or the base of a fingernail.
- Check the airway and breathing.Clear the mouth or nose of any blockage. Check breathing for 10 seconds. Is the chest moving? Can you hear breathing sounds? Can you feel breathing on your cheek?
- Check for signs of overdose, such as: no response to touch or sound, slow and irregular breathing or no breathing, snoring, gasping, or rapid breathing, blue-tinged fingers or lips, very small pupils.
- If overdose is suspected, Nyxoid should be administered as soon as possible.
- Call an ambulance.Nyxoid is not a substitute for emergency medical care.
|
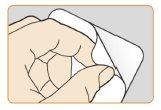
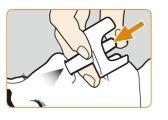
- Peel offthe back of the blister by a corner to remove the nasal sprayfrom the container. Place the nasal spray in an easily accessible location.
|
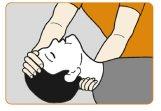
- Turn the patient onto their back. Hold the neck from behind, allowing the head to tilt back. Clear anything that may block the nose.
|
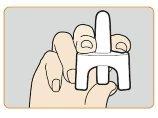
- Hold the lower part of the plunger on the nasal spray device with your thumb and place your index and middle fingers on either side of the nozzle. Do not prime or test the device before useas it contains only one dose and cannot be reused.
- Gently insert the nozzle of the device into one of the nostrils. Firmly pressthe plunger until it clicksto administer the dose. Remove the nozzle of the nasal spray device from the nostril after administering the dose.
- Place the patient in the recovery positionon their side, with the mouth
open, facing downwards, and stay with the patient until emergency services arrive. Check if there is any improvement in the patient's breathing, level of alertness, and response to noise or touch.
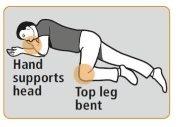
The hand | ||
holds the | ||
head | ||
Top part | ||
of the bent leg | ||
- If the patient does not improvein 2-3 minutes, a second dose can be administered. Note that even if they wake up, they may become unconscious again and stop breathing. If this happens, a second dose can be administered immediately. Administer Nyxoid in the other nostril using a new Nyxoid nasal spray device. This can be done whilethe patient is in the recovery position.
- If the patient does not respond to two doses, other doses can be administered (if available). Stay with the patient and continue to check for any improvement until emergency services arrive, who will administer further treatment.
In patients who are unconscious and not breathing normally, when possible, additional life-support measures should be applied.
For more information or videos, scan the QR code or visit www.nyxoid.com
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may occur with this medicine.
Medical conditions to be aware of
Nyxoid may cause acute withdrawal symptomsif the patient is dependent on opioids. The symptoms can include: The drug withdrawal syndrome includes restlessness, irritability, hyperesthesia (increased skin sensitivity), nausea (feeling sick), vomiting (being sick), gastrointestinal pain (stomach cramps), muscle spasms (sudden muscle tension), dysphoria (unpleasant or uncomfortable mood), insomnia (difficulty sleeping), anxiety, hyperhidrosis (excessive sweating), piloerection (goosebumps, shivers, or tremors), tachycardia (rapid heart rate), increased blood pressure, yawning, pyrexia (fever). Behavioral changes, such as violent behavior, nervousness, and excitement, may also occur.
Acute withdrawal symptoms occur infrequently (may affect up to 1 in
- people).
Tell your doctorif you experience any of these symptoms.
Very common: may affect more than 1 in 10 people
- Feeling sick (nausea)
Common: may affect up to 1 in 10 people
- Dizziness, headache
- Fast heart rate
- High blood pressure, low blood pressure
- Vomiting
Uncommon: may affect up to 1 in 100 people
- Tremor
- Slow heart rate
- Sweating
- Irregular heart rate
- Diarrhea
- Dry mouth
- Rapid breathing
Rare: may affect up to 1 in 10,000 people
- Allergic reactions, such as swelling of the face, mouth, lips, or throat, anaphylactic shock
- Irregular and potentially life-threatening heartbeats, myocardial infarction
- Fluid accumulation in the lungs
- Skin problems such as itching, rash, redness, swelling, or intense skin peeling
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Nyxoid
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, blister, and label after EXP. The expiry date is the last day of the month shown.
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
Nyxoid composition
- The active ingredient is naloxone. Each nasal spray contains 1.8 mg of naloxone (as dihydrochloride dihydrate).
- The other ingredients are trisodium citrate dihydrate (E331), sodium chloride, hydrochloric acid (E507), sodium hydroxide (E524), and purified water (See "Nyxoid contains sodium" in section 2).
Appearance of the product and container contents
This medication contains naloxone in 0.1 ml of a clear, colorless to pale yellow solution in a pre-loaded nasal spray, solution in a single-dose container (nasal spray, solution)
Nyxoid is packaged in a cardboard box containing 2 closed nasal sprays in individual blisters. Each nasal spray contains a single dose of naloxone.
Marketing authorization holder
Mundipharma Corporation (Ireland) Limited
United Drug House Magna Drive
Magna Business Park
Citywest Road
Dublin 24
Ireland
Manufacturer
Mundipharma DC B.V.
Leusderend 16
3832 RC Leusden
Netherlands
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium | Lithuania | |||
Mundipharma Comm. VA | Mundipharma Corporation (Ireland) Limited | |||
+32 2 358 54 68 | Ireland | |||
Tel +353 1 206 3800 | ||||
Luxembourg | ||||
Bulgarian representative | Mundipharma Comm. VA | |||
Phone: + 359 2 962 13 56 | +32 2 358 54 68 | |||
e-mail: [email protected] | ||||
Czech Republic | Hungary |
Mundipharma GesmbH Austria - organizational unit | Medis Hungary Kft |
Phone: + 420 296 188 338 | Phone: +36 23 801 028 |
E-Mail: [email protected] | |
Denmark | Malta |
Mundipharma A/S | Mundipharma Corporation (Ireland) Limited |
Phone: +45 17 48 00 | Ireland |
Phone: +353 1 206 3800 |
Germany | Netherlands | ||
Mundipharma GmbH | Mundipharma Pharmaceuticals B.V. | ||
Toll-free info line: +49 69 506029-000 | Phone: + 31 (0)33 450 82 70 | ||
Estonia | Norway |
Mundipharma Corporation (Ireland) Limited | Mundipharma AS |
Ireland | Phone: + 47 67 51 89 00 |
Phone: +353 1 206 3800 |
Greece | Austria | |
Mundipharma Corporation (Ireland) Limited | Mundipharma Gesellschaft m.b.H. | |
Ireland | Phone: +43 (0)1 523 25 05 | |
Phone: +353 1 206 3800 |
Spain | Poland | ||
Mundipharma Pharmaceuticals, S.L. | Mundipharma Polska Sp. z o.o. | ||
Phone: +34 91 3821870 | Phone: + (48 22) 3824850 | ||
France | Portugal | ||
MUNDIPHARMA SAS | Mundipharma Farmacêutica Lda | ||
Phone: +33 1 40 65 29 29 | Phone: +351 21 901 31 62 | ||
Croatia | Romania | ||
Medis Adria d.o.o. | Mundipharma Gesellschaft m.b.H., Austria | ||
Phone: + 385 (0) 1 230 34 46 | Phone: +40751 121 222 | ||
Ireland | Slovenia | |
Mundipharma Pharmaceuticals Limited | Medis, d.o.o. | |
Phone: +353 1 206 3800 | Phone: +386 158969 00 | |
Iceland | Slovak Republic | |
Icepharma hf. | Mundipharma Ges.m.b.H.-o.z. | |
Phone: + 354 540 8000 | Phone: + 4212 6381 1611 | |
Italy | Finland | ||
Mundipharma Pharmaceuticals Srl | Mundipharma Oy | ||
Phone: +39 02 3182881 | Phone: + 358 (0)9 8520 2065 | ||
Cyprus | Sweden | ||
Mundipharma Pharmaceuticals Ltd | Mundipharma AB | ||
Phone: +357 22 815656 | Phone: + 46 (0)31 773 75 30 | ||
Latvia | ||
SIA Inovativo biomedicinas tehnologiju instituts | ||
Phone: + 37167800810 | ||
Date of the last revision of this prospectus:
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NYXOID 1.8 mg NASAL SPRAY SOLUTION SINGLE-DOSE CONTAINERDosage form: INJECTABLE, 0.4 mg/mlActive substance: naloxoneManufacturer: Medochemie LimitedPrescription requiredDosage form: INJECTABLE, 0.4 mg/mlActive substance: naloxoneManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.4 mg/mlActive substance: naloxoneManufacturer: B. Braun Melsungen AgPrescription required
Online doctors for NYXOID 1.8 mg NASAL SPRAY SOLUTION SINGLE-DOSE CONTAINER
Discuss questions about NYXOID 1.8 mg NASAL SPRAY SOLUTION SINGLE-DOSE CONTAINER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions