
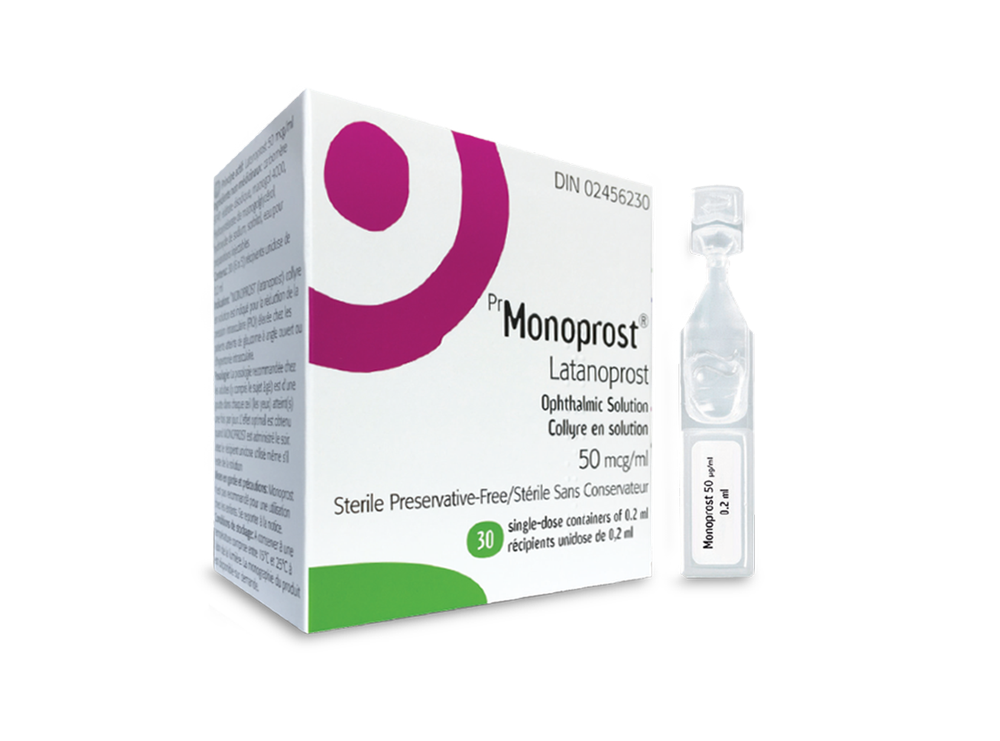
Monoprost

Ask a doctor about a prescription for Monoprost

How to use Monoprost
Package Leaflet: Information for the Patient
MONOPROST, 50 micrograms/ml, eye drops, solution in a single-dose container
Latanoprost
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Contents of the Package Leaflet
- 1. What Monoprost is and what it is used for
- 2. Important information before using Monoprost
- 3. How to use Monoprost
- 4. Possible side effects
- 5. How to store Monoprost
- 6. Contents of the pack and other information
1. WHAT MONOPROST IS AND WHAT IT IS USED FOR
Monoprost belongs to a group of medicines known as prostaglandins. The medicine lowers the pressure in the eye by increasing the natural outflow of aqueous fluid from the eye into the bloodstream. Monoprost is used to treat a condition called open-angle glaucomaand a condition called ocular hypertension. Both conditions are associated with high pressure in the eye and can affect your vision.
2. IMPORTANT INFORMATION BEFORE USING MONOPROST
When not to use Monoprost:
- If you are allergic (hypersensitive) to latanoprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Monoprost, tell your doctor, pharmacist, or nurse if you have any of the following:
- If you have had eye surgery (including cataract surgery) or are planning to have it.
- If you have eye problems (such as eye pain, inflammation, or irritation, blurred vision).
- If you have severe or uncontrolled asthma.
- If you wear contact lenses. You can use Monoprost, but follow the instructions for contact lens wearers in section 3.
- If you have had or have a viral infection of the eye caused by the Herpes simplexvirus (HSV).
Children
Monoprost has not been studied in children under 18 years of age.
Monoprost and other medicines
Monoprost may interact with other medicines. Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and any you plan to take.
Pregnancy and breastfeeding
Do not use Monoprostduring pregnancy or breastfeeding. If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
While using Monoprost, you may experience temporary blurred vision. If this happens, do not driveor operate machinery until the symptoms have resolved.
Important information about some of the ingredients of Monoprost
Monoprost contains macrogolglycerol hydroxystearate 40 (derived from castor oil), which may cause skin reactions.
3. HOW TO USE MONOPROST
Normally used dose
- Use Monoprost exactly as your doctor has told you. If you are unsure, ask your doctor or pharmacist.
- The recommended dose for adults (including the elderly) is one drop into the affected eye(s) once daily. The best time to administer the dose is in the evening.
- Do not use Monoprost more than once a day, as this may reduce the effectiveness of the treatment.
- Use Monoprost exactly as described in this leaflet or as your doctor has told you, for as long as your doctor recommends. If you are unsure, ask your doctor, pharmacist, or nurse.
People wearing contact lenses
If you wear contact lenses, remove them before using Monoprost. Wait 15 minutes after using Monoprost before putting your contact lenses back in.
Instructions for use
The drops come in single-dose containers. The solution from one single-dose container of Monoprost must be used immediately after opening and administered by dropping into the affected eye(s). Since the sterility of the solution cannot be maintained after the single-dose container is opened, a new single-dose container must be opened for each use.
- 1. Wash your hands, stand or sit comfortably.
- 2. Open the sachet. Write the date of first opening on the sachet.
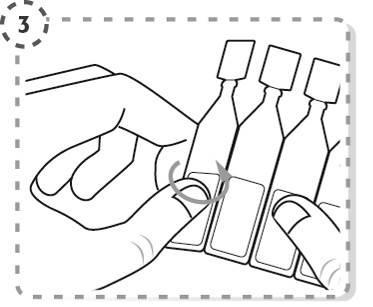
- 3. Break off one single-dose container from the strip.
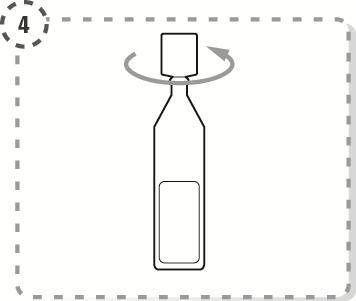
- 4. Twist off the top of the single-dose container, as shown in the picture. Do not touch the tip of the container after opening.
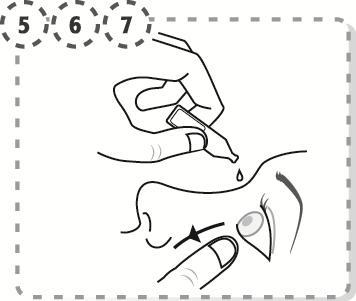
- 5. Gently pull down the lower lid of the affected eye.
- 6. Place the tip of the single-dose container close to the eye, but not touching the eye surface.
- 7. Gently squeeze the single-dose container to release one drop into the eye, then release the lower lid.
- 8. Close the eye and press the inner corner of the eye with your finger for 1 minute, keeping the eye closed.
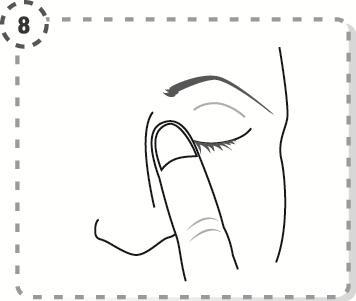
- 9. Repeat the steps for the other eye, if your doctor has told you to do so. Each single-dose container contains enough solution to treat both eyes.
- 10. Discard the single-dose container after use. Do not store it for reuse. Since the sterility of the solution cannot be maintained after the single-dose container is opened, a new single-dose container must be opened for each use.
Using Monoprost with other eye drops
If you are using other eye drops, wait at least 5 minutes between using Monoprost and the other eye drops.
Using more Monoprost than prescribed
If you accidentally use more eye drops than prescribed, you may experience moderate eye irritation with redness and tearing. These symptoms should resolve, but if you are concerned, you should contact your doctor for advice. If you accidentally swallow Monoprost, contact your doctor immediately.
Missing a dose of Monoprost
Take the dose at the usual time. Do not take a double dose to make up for a missed dose. If you are unsure, ask your doctor or pharmacist.
Stopping Monoprost treatment
If you want to stop using Monoprost, talk to your doctor. If you have any further questions on the use of this product, ask your doctor, pharmacist, or nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Monoprost can cause side effects, although not everybody gets them. The following side effects have been reported with Monoprost:
Very common: may affect more than 1 in 10 people
- Gradual change in eye color due to an increase in the amount of brown pigment in the colored part of the eye called the iris.
- This effect is more frequently observed in patients with mixed-colored irides (blue-brown, grey-brown, yellow-brown, or green-brown) than in patients with uniformly colored eyes (blue, grey, green, or brown).
- The change in eye color may take years to develop, but it is usually noticeable within 8 months of treatment.
- The change in eye color may be permanent and may be more noticeable if Monoprost is used in one eye only.
- No symptoms or signs other than the change in eye color have been associated with this effect.
- The change in iris color does not seem to worsen after stopping Monoprost.
- Eye redness.
- Eye irritation (feeling of burning, grittiness, itching, stinging, or the sensation of having something in the eye).
- Gradual change in the appearance of the eyelashes and eyelid hair of the treated eye (particularly in patients of Japanese descent). These changes include increased pigmentation (darkening), length, thickness, and number of eyelashes.
Common: may affect up to 1 in 10 people
- Eye surface disorders, eyelid inflammation, eye pain, sensitivity to light (photophobia), conjunctivitis.
Uncommon: may affect up to 1 in 100 people
- Swelling of the eyelids, dry eyes, inflammation or irritation of the eye surface (cornea), blurred vision, inflammation of the colored part of the eye (uveitis), swelling of the macula (macular edema).
- Skin rash.
- Chest pain (angina), awareness of heartbeat (palpitations).
- Asthma, shortness of breath (dyspnea).
- Chest pain.
- Headache, dizziness.
- Muscle pain, joint pain.
- Feeling sick (nausea), vomiting.
Rare: may affect up to 1 in 1,000 people
- Inflammation of the iris (iritis), symptoms of swelling, feeling of something in the eye or damage to the eye surface, swelling around the eye, changes in the direction of eyelash growth or the appearance of a double row of eyelashes, accumulation of fluid within the colored part of the eye (iris cyst).
- Skin reactions on the eyelids, darkening of the eyelid skin.
- Worsening of asthma.
- Severe itching of the skin.
- Development of a viral infection of the eye caused by the Herpes simplexvirus (HSV).
Very rare: may affect up to 1 in 10,000 people
- Worsening of angina in patients with a history of angina.
- Appearance of sunken eyes (deepening of the eyelid sulcus).
Reporting of side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. You can also report side effects directly to the Pharmacovigilance Department of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products – Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl. You can also report side effects to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE MONOPROST
Keep the medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the carton, sachet, and single-dose container. The expiry date refers to the last day of that month. Store in a temperature below 25°C. After opening the sachet: use the single-dose containers within 10 days. After opening the single-dose container: use immediately and discard the single-dose container after use. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Monoprost contains
The active substance is latanoprost. 1 ml of eye drops contains 50 micrograms of latanoprost. The other ingredients are macrogolglycerol hydroxystearate 40, sorbitol, carbomer 974P, macrogol 4000, disodium edetate, sodium hydroxide (for pH adjustment), water for injections.
What Monoprost looks like and contents of the pack
Monoprost is an eye drop solution in a single-dose container. The solution is slightly yellow, opalescent, and does not contain preservatives. Monoprost is available in single-dose containers, packaged in sachets of 5 or 10. Each single-dose container contains 0.2 ml of solution. Pack sizes: 30 (6 x 5), 30 (3 x 10), 90 (18 x 5), or 90 (9 x 10) single-dose containers. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Laboratoires THEA, 12, rue Louis Blériot, 63017 Clermont-Ferrand Cedex 2, France
Manufacturer:
EXCELVISION, 27, rue de la Lombardière, ZI la Lombardière, 07100 Annonay, France, Laboratoires THEA, 12, rue Louis Blériot, 63017 Clermont-Ferrand Cedex 2, France, LABORATOIRE UNITHER, 1 rue de l’Arquerie, 50200 Coutances, France, FAREVA Mirabel, Route de Marsat, Riom, 63693 Clermont-Ferrand Cedex 9, France
For further information, contact the marketing authorization holder:
Thea Polska Sp. z o.o., ul. Cicha 7, 00-353 Warsaw, Tel. 22 642 87 77
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium, Bulgaria, Cyprus, Denmark, Finland, France, Greece, Spain, Netherlands, Iceland, Latvia, Luxembourg, Germany, Norway, Poland, Portugal, Sweden, Italy ................................ Monoprost, Ireland ........................................................................................................................ Monopost Unidose, Austria, Czech Republic, Lithuania, Romania, Slovakia, Slovenia, United Kingdom ................................ Monopost, Estonia ....... ..................................................................................................................................Monopro
Date of last revision of the leaflet: 01-05-2025
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterEXCEL VISION Laboratoires THEA Laboratoires Unither
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MonoprostDosage form: Drops, 50 mcg/mlActive substance: latanoprostManufacturer: Pharmaselect International Beteiligungs GmbHPrescription requiredDosage form: Drops, 50 mcg/mlActive substance: latanoprostManufacturer: Jadran-Galenski laboratorij d.d.Prescription requiredDosage form: Drops, 0.05 mg/mlActive substance: latanoprostManufacturer: S.C. Rompharm Company S.R.L.Prescription required
Alternatives to Monoprost in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Monoprost in Испания
Alternative to Monoprost in Украина
Online doctors for Monoprost
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Monoprost – subject to medical assessment and local rules.














