
LATANOPROST STADAFARMA 50 micrograms/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS

How to use LATANOPROST STADAFARMA 50 micrograms/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Latanoprost Stadafarma 50 micrograms/ml eye drops, solution in single-dose container
Read this entire leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Latanoprost Stadafarma and what is it used for
- What you need to know before you start using Latanoprost Stadafarma
- How to use Latanoprost Stadafarma
- Possible side effects
- Storage of Latanoprost Stadafarma
- Contents of the pack and further information
1. What is Latanoprost Stadafarma and what is it used for
Latanoprost Stadafarma belongs to a group of medicines known as prostaglandin analogues. It works by increasing the natural outflow of fluid from the inside of the eye into the bloodstream.
Latanoprost Stadafarma is used to treat conditions known as open-angle glaucomaand ocular hypertension. Both of these conditions are related to an increase in pressure within the eye, which can lead to problems with your sight.
2. What you need to know before you start using Latanoprost Stadafarma
Do not useLatanoprost Stadafarma
- If you are allergic (hypersensitive) to latanoprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
If you think any of the following apply to you, talk to your doctor or pharmacist before you start using latanoprost:
- If you have had or are going to have eye surgery (including cataract surgery).
- If you have eye problems (such as eye pain, irritation or inflammation, blurred vision).
- If you have dry eyes.
- If you have severe asthma or uncontrolled asthma.
- If you wear contact lenses. You can continue to use latanoprost, but you should follow the instructions for contact lens wearers in section 3.
- If you have had or are having a viral infection of the eye caused by the herpes simplex virus (HSV).
Children
This medicine has not been studied in children (under 18 years of age).
Other medicines and Latanoprost Stadafarma
Latanoprost may interact with other medicines. Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Pregnancy and breastfeeding
Do not uselatanoprost if you are pregnant or breastfeeding, unless your doctor considers it necessary.
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
When using latanoprost, you may experience blurred vision for a short time. If this happens, do not driveor use tools or machines until your vision is clear again.
Latanoprost Stadafarma contains phosphates
This medicine contains 6.3 mg/ml of phosphates, which is equivalent to 0.24 mg/drop.
If you have severe damage to the clear layer on the front of your eye (the cornea), treatment with phosphates, in very rare cases, may cause cloudy patches on the cornea due to calcium deposits formed during treatment.
3. How to use Latanoprost Stadafarma
- Follow the instructions for administration of latanoprost exactly as told by your doctor. Ask your doctor or pharmacist if you are not sure.
- The recommended dose for adults (including elderly patients) is one drop into the affected eye(s) once daily. It is best to administer it in the evening.
- Do not use latanoprost more than once daily; the effectiveness of the treatment may decrease if it is administered more frequently.
- Use latanoprost as your doctor has told you until they tell you to stop.
Contact lens wearers
If you wear contact lenses, you should remove them before using latanoprost. After applying latanoprost, wait 15 minutes before putting your contact lenses back in.
Instructions for use
Each carton contains 3 or 9 bags. Open only 1 bag at a time. Use all the single-dose containers before opening a new bag.
Separate the single-dose container from the strip before use.
- Wash your hands and sit or stand comfortably.
- Gently pull down the lower eyelid of the affected eye.
- Place the tip of the single-dose container close to the eye, but without touching it.
- Gently squeeze the single-dose container so that only one drop falls into the eye, then release the lower eyelid.
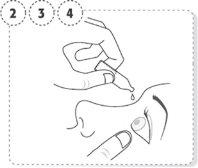
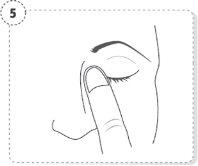
- Press the finger on the edge of the affected eye, near the nose. Apply pressure for 1 minute, keeping your eye closed.
- Repeat in the other eye, if your doctor has told you to do so.
- After use, discard the single-dose container. Do not store it for later use.
If you use Latanoprost Stadafarma with other eye drops
Wait at least 5 minutes between the application of latanoprost and the administration of other eye drops.
If you use more Latanoprost Stadafarma than you should
If you have applied more drops in the eye than you should, you may feel a slight irritation in the eye and your eyes may become red and watery. This should pass, but if you are concerned, contact your doctor.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicological Information Service, phone 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Latanoprost Stadafarma
Continue with the next dose as usual. Do not apply an extra drop in the eye to make up for the missed dose. If you have any doubts, consult your doctor or pharmacist.
If you stop using Latanoprost Stadafarma
If you want to stop using latanoprost, consult your doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following are known side effects of this medicine:
Very common side effects(may affect more than 1 in 10 people):
- Gradual change in eye color due to an increase in the amount of brown pigment in the colored part of the eye, known as the iris.
- If you have mixed-color eyes (blue-brown, grey-brown, yellow-brown, or green-brown), you are more likely to experience this change than if your eyes are a single color (blue, grey, green, or brown).
- The change in eye color takes years to develop, although it can usually be seen after 8 months of treatment.
- The change in eye color may be permanent and may be more noticeable if this medicine is used in only one eye.
- The change in eye color does not seem to be associated with any problems.
- The change in eye color does not progress once treatment with this medicine has been stopped.
- Redness of the eye.
- Ocular irritation (feeling of burning, feeling of grit in the eye, itching, pain, and feeling of a foreign body in the eye). If you experience severe ocular irritation that makes your eyes water excessively or makes you consider stopping treatment, consult your doctor, pharmacist, or nurse as soon as possible (within a week). You may need to have your treatment reviewed to ensure you are receiving the right treatment for your condition.
- Gradual change in the eyelashes of the treated eye and the fine hair around the treated eye, mainly observed in patients of Japanese origin. These changes include an increase in color (darkening), length, thickness, and number of eyelashes.
Common side effects(may affect up to 1 in 10 people):
- Irritation or erosion of the surface of the eye, inflammation of the eyelid (blepharitis), eye pain, and sensitivity to light (photophobia), conjunctivitis.
Uncommon side effects(may affect up to 1 in 100 people):
- Swelling of the eyelids, dry eye, inflammation or irritation of the surface of the eye (keratitis), blurred vision, inflammation of the colored part of the eye (uveitis), swelling of the retina (macular edema).
- Skin rash.
- Chest pain (angina), feeling your heartbeat (palpitations).
- Asthma, difficulty breathing (dyspnea).
- Chest pain.
- Headache, dizziness.
- Muscle pain, joint pain.
Rare side effects(may affect up to 1 in 1,000 people):
- Inflammation of the iris (iritis), symptoms of swelling or injury/damage to the surface of the eye, swelling around the eye (periorbital edema), misdirected eyelashes or extra row of eyelashes, scarring of the surface of the eye, accumulation of fluid in the colored part of the eye (iris cyst).
- Skin reactions on the eyelids, darkening of the skin of the eyelids.
- Worsening of asthma.
- Severe itching of the skin.
- Development of a viral infection of the eye caused by the herpes simplex virus (HSV).
Very rare side effects(may affect up to 1 in 10,000 people):
- Worsening of angina in patients who also have heart problems, appearance of sunken eyes (greater depth of the eyelid sulcus).
In very rare cases, some patients with severe damage to the clear layer on the front of the eye (cornea) have developed cloudy patches on the cornea due to calcium deposits formed during treatment.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Monitoring System for Human Use. https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Latanoprost Stadafarma
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, on the bag, and on the single-dose container. The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
Keep the bag in the carton after opening to protect from light.
After first opening of the bag: use the single-dose containers within 20 days.
After first opening of the single-dose container: use immediately and discard the single-dose container after use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Composition of Latanoprost Stadafarma
- The active substance is latanoprost 50 micrograms/ml.
- The other ingredients are carmellose sodium, D-mannitol, tyloxapol, sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, hydrochloric acid (for pH adjustment), water for injections.
Appearance of the product and contents of the pack
Latanoprost Stadafarma 50 micrograms/ml eye drops is a colorless or pale yellow solution in a single-dose container.
The single-dose containers are packaged in bags of 10 units (2 strips of 5 single-dose containers), each single-dose container contains 0.2 ml of solution.
The cartons contain 30 single-dose containers (3 bags with 10 single-dose containers) or 90 single-dose containers (9 bags with 10 single-dose containers).
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
Spain
Manufacturer
Pharma Stulln GmbH
Werksstrasse 3
92551 Stulln, Germany
This medicine is authorised in the Member States of the European Economic Area under the following names:
Germany Latanoprost AL 50 Mikrogramm/ml Augentropfen, Lösung im Einzeldosisbehältnis
Date of last revision of this leaflet:May 2024
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LATANOPROST STADAFARMA 50 micrograms/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYE DROP, 50 micrograms/mlActive substance: latanoprostManufacturer: Santen OyPrescription requiredDosage form: EYE DROP, 50 µg/mlActive substance: latanoprostManufacturer: Esteve Pharmaceuticals S.A.Prescription requiredDosage form: EYEDROP, 50 MG/MLActive substance: latanoprostManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for LATANOPROST STADAFARMA 50 micrograms/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about LATANOPROST STADAFARMA 50 micrograms/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















