
Miofree

Ask a doctor about a prescription for Miofree

How to use Miofree
Leaflet attached to the packaging: patient information
Miofree, 100 micrograms/ml, eye drops, solution
Atropine sulfate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Miofree and what is it used for
- 2. Important information before using Miofree
- 3. How to use Miofree
- 4. Possible side effects
- 5. How to store Miofree
- 6. Contents of the pack and other information
1. What is Miofree and what is it used for
Miofree contains atropine sulfate as the active substance.
The Miofree eye drops are used to slow down the development of myopia in children and adolescents aged 6 to 18 years.
In justified cases, the doctor may decide to start treatment at an earlier age.
Miofree does not contain preservatives.
2. Important information before using Miofree
When not to use Miofree:
- if the patient is allergic to atropine sulfate or other substances with the same action (called anticholinergics), or any of the other ingredients of this medicine (listed in section 6);
- if the patient has primary glaucoma (increased pressure in the eyes) with a tendency to angle closure;
- if the patient has glaucoma with a narrow angle.
Warnings and precautions
Before starting treatment with Miofree, discuss it with your doctor or pharmacist.
- Treatment should be supervised by an ophthalmologist specializing in the treatment of children and adolescents. During treatment, especially long-term, the ophthalmologist will perform regular eye exams, including monitoring the development of the anterior segment of the eye, intraocular pressure, retinal condition, and myopia development.
- In children with Down syndrome, spastic paralysis, or brain damage, atropine should be used with caution, as increased sensitivity to atropine has been reported.
- After administering atropine, protect the eyes from light - therefore, the medicine should be instilled in the evening, just before bedtime.
- The drops, due to the type of active substance, may temporarily worsen vision, and the child may have difficulty with sharp vision at close range or while reading, or may cause photophobia - in such cases, you should contact your doctor to discuss possible solutions to this problem.
- Care should be taken in patients with accelerated heart rate (tachycardia).
- Miofree is intended for use in children and adolescents aged 6 to 18 years - see section 1 of the leaflet.
- If the patient uses soft contact lenses, they should not instill Miofree while wearing contact lenses. After instilling the medicine, wait 15 minutes before putting in the lenses.
- Patients who have had contact allergy to silver in the past should not use this medicine, as the drops may contain trace amounts of silver from the bottle.
When instilling, be very careful not to get the medicine into the mouth. After use, wash your hands (see also section 3, subsection "Method of administration").
Always discuss any problems or difficulties during or after using Miofree eye drops with your doctor.
Miofree and other medicines
Tell your doctor or pharmacist about all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to use.
- The action of atropine is enhanced by medicines with anticholinergic action, such as amitriptyline (an antidepressant), haloperidol, and chlorpromazine (medicines used in mental disorders), hydroxyzine, clemastine (antihistamines belonging to the so-called first-generation antihistamines), and some medicines used in Parkinson's disease.
- When using certain glaucoma medications (cholinergic glaucoma medications with prolonged action) with atropine, such as ecotiotropate, the anti-glaucoma and pupil-constricting effects may be inhibited. Concurrent use of atropine may also inhibit the action of these medications when used to treat strabismus. Although there are currently no studies evaluating the use of low doses of atropine in eye drops in children with increased intraocular pressure, there is some data suggesting that the use of low doses of atropine in eye drops may reduce the risk of glaucoma in children with myopia.
- When atropine and physostigmine, carbachol, or pilocarpine (medicines used, for example, in the treatment of glaucoma) are used together, there is mutual neutralization of the action of the medicines.
- If there is significant systemic absorption of atropine sulfate administered to the eye (which is unlikely):
- when using potassium citrate, products containing potassium, or medicines used in myasthenia, side effects and symptoms of toxicity may occur.
- when using medicines that affect the central nervous system, such as antiemetics, phenothiazine derivatives, or barbiturates, muscle spasms of the neck and back (opisthotonus), seizures, coma, and extrapyramidal symptoms may occur.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Miofree is intended for children and adolescents (see section 1 of the leaflet). The data presented below concerns the active substance - atropine sulfate (atropine).
Atropine may be used during pregnancy only if, in the doctor's opinion, the benefit to the mother outweighs the potential risk to the fetus.
It is not known whether atropine sulfate passes into breast milk.
Caution should be exercised when using atropine sulfate in breastfeeding women.
There are no data on the effects on fertility in humans.
Driving and using machines
Miofree is intended for children and adolescents (see section 1 of the leaflet). The data presented below concerns the active substance - atropine sulfate (atropine).
Clinical trials have shown that the effect of 0.01% atropine eye drops on visual acuity was very small (in 2.3% of patients). Possible side effects, such as blurred vision, may affect some patients' ability to drive or operate machinery. The possible effect of Miofree on the ability to drive or operate machinery should be assessed, especially at the beginning of treatment.
3. How to use Miofree
This medicine should always be used exactly as prescribed by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist.
Treatment should be supervised by an ophthalmologist specializing in the treatment of children and adolescents, who will determine the duration of treatment based on regular clinical assessment. It is essential to use Miofree exactly as prescribed by your doctor.
Children and adolescents aged 6 to 18 years
Instill 1 drop of the medicine into the conjunctival sac of each eye in the evening, just before bedtime.
Do not use more drops than prescribed by your doctor or more frequently than prescribed.
Using the medicine more often than recommended or in larger doses will not lead to faster improvement and may cause or worsen side effects.
When instilling, be very careful not to get the medicine into the mouth.
If other eye drops are used in addition to Miofree, wait at least 10 minutes between instilling Miofree and using other drops. Eye ointments should be used last.
Method of administration
Miofree is intended for external use only - it should be used locally in the eye (eyes).
Follow the instructions below to use Miofree correctly.
- 1. Wash your hands thoroughly.
- 2. Remove the protective cap (Figure 1).
- 3. Hold the bottle with the tip down (vertically), with your thumb on the pump cap and your other fingers on the bottom of the bottle (Figure 2). Before first use, press the pump cap 17 times to achieve a consistent drop size.
- 4. Tilt your head back and gently pull down the lower eyelid to create a pocket between the eye and the eyelid.
- 5. Place the tip of the bottle close to the eye without touching it.
- 6.
Do not touch the dropper to the eye, eyelid, or surrounding areas, as this may cause infection.
- 7. Hold the bottle as described above (Figure 2), in a vertical position over the eye, and gently press the pump cap to instill one drop into the eye, then release the lower eyelid (Figure 3).
- 8. Press your finger to the corner of the affected eye near the nose (Figure 4). Hold for about 2 minutes, keeping the eye closed.
- 9. Instill the medicine into the other eye, if prescribed by your doctor.
- 10. Immediately after use, put the protective cap back on the bottle tip.
- 11. If a drop misses the eye, try again.
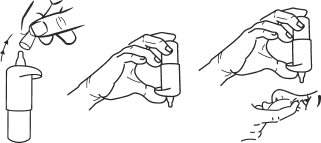
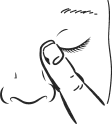
Figure 1.
Figure 2.
Figure 3.
Figure 4.
After using the medicine, wash your hands.
Only use one bottle at a time.
The ophthalmologist will decide when to stop using the medicine and provide guidance on how to gradually discontinue it.
Using more Miofree than prescribed
In case of excessive systemic absorption of atropine sulfate, symptoms such as: balance disorders, behavioral disorders, dizziness, accelerated heart rate, irregular heartbeat, fever, hallucinations, and rash may occur. There may also be: skin redness and dryness, increased thirst, dryness in the mouth, drowsiness, fatigue, weakness, dry skin, and urinary retention. In addition, the following may occur: skin rash (eczema), severe generalized reactions with decreased blood pressure and worsening respiratory disorders.
If these symptoms occur or the medicine has been used incorrectly (e.g., swallowed), contact a doctor immediately, who will provide appropriate treatment.
Missing a dose of Miofree
If a dose is missed, it should be taken as soon as possible. If it is almost time for the next dose, skip the missed dose and take the next one according to the established schedule. Do not take a double dose to make up for the missed dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following serious side effects occur, contact a doctor or go to the emergency department of the nearest hospital immediately:
- redness and dryness of the skin, rapid and irregular heartbeat, fever, mental disorders, psychosis, and loss of nerve-muscle coordination;
- allergic reactions, including rash, swelling of the face, lips, tongue, and/or throat, which may cause difficulty breathing or swallowing; The frequency of these side effects is described below.
Consult a doctor if any of the following side effects occur.
Side effects related to the use of 0.01% atropine eye drops
Common (may affect up to 1 in 10 people):
- allergy, photophobia (increased sensitivity to light), blurred vision, decreased visual acuity.
Frequency not known (frequency cannot be estimated from the available data):
- contact dermatitis, eczematous dermatitis;
- local irritation, nodular conjunctivitis, hyperemia, eye edema, discharge.
Side effects related to the use of 1% atropine eye drops (100 times higher concentration than in Miofree) or higher
Very rare (may affect up to 1 in 10,000 people):
- confusional state, dizziness (especially in the elderly), vomiting.
Frequency not known (frequency cannot be estimated from the available data):
- hypersensitivity (manifested by skin rash or conjunctivitis);
- psychiatric disorders, anxiety, agitation, hallucinations, coordination disorders, severe ataxia (coordination disorder);
- transient stinging, edema, and conjunctivitis (may occur after prolonged use of atropine); vision and accommodation disorders (ability to adapt the eye to viewing objects at different distances), increased intraocular pressure, (especially in patients with glaucoma with a narrow angle), conjunctivitis, itching (itching) of the eyes, eyelid edema, lacrimation;
- rapid and irregular heartbeat (palpitations), cardiac arrhythmias (e.g., atrial fibrillation), transient bradycardia (slow heart rate) followed by tachycardia, hypotension with progressive breathing difficulties, decreased bronchial secretion;
- infantile colic, dryness in the mouth (causing difficulty swallowing and speaking), nausea, constipation;
- skin redness and dryness, urinary urgency, urinary retention, elevated body temperature.
Side effects related to the use of 0.01% to 1% atropine eye drops
Frequency not known (frequency cannot be estimated from the available data):
- psychosis, decreased consciousness, headache, worsening of seizure attacks, bilateral pigment dispersion syndrome (characterized by the penetration of pigment granules into the aqueous humor of the eye), accelerated heart rate, fatigue.
During treatment, other general side effects and symptoms listed in the "Using more Miofree than prescribed" section may occur.
Reporting side effects
If any side effects occur, including any side effects not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Miofree
Keep the medicine out of sight and reach of children.
Do not store in the refrigerator or freeze.
After opening the bottle:
Do not store above 30°C.
Do not store in the refrigerator or freeze.
2.5 ml:
Shelf life after opening the bottle: 30 days.
7.5 ml:
Shelf life after opening the bottle: 90 days.
Do not use this medicine after the expiry date stated on the carton and bottle.
The expiry date stated is the last day of the given month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Miofree contains
- The active substance of the medicine is atropine sulfate. Each ml of solution contains 100 micrograms of atropine sulfate. Each 2.5 ml bottle contains at least 60 drops. Each 7.5 ml bottle contains at least 180 drops.
- The other ingredients are: sodium chloride, disodium edetate, sodium hydroxide (to adjust pH), diluted hydrochloric acid (to adjust pH), purified water.
What Miofree looks like and contents of the pack
Miofree is a sterile eye drop solution in the form of a clear, colorless solution.
The packaging consists of a semi-transparent HDPE bottle with a multidose dropper with a pump (PP, HDPE, LDPE), an HDPE cap, and a dosing aid cap (PP).
The carton contains one bottle containing 2.5 ml or 7.5 ml of solution.
Not all pack sizes may be marketed.
Marketing authorization holder
Polpharma S.A.
Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
Jadran - Galenski Laboratorij d.d.
Svilno 20
51000 Rijeka
Croatia
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterJadran-Galenski laboratorij d.d.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MiofreeDosage form: Drops, 10 mg/mlActive substance: atropineManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription requiredDosage form: Drops, 10 mg/mlActive substance: cyclopentolateDosage form: Solution, (0.2 mg + 3.1 mg + 10 mg)/mlActive substance: tropicamide, combinationsManufacturer: Delpharm Tours Laboratoires THEAPrescription required
Alternatives to Miofree in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Miofree in Ukraine
Alternative to Miofree in Spain
Online doctors for Miofree
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Miofree – subject to medical assessment and local rules.














