Miflonide Breezhaler

Ask a doctor about a prescription for Miflonide Breezhaler

How to use Miflonide Breezhaler
PATIENT INFORMATION LEAFLET: USER INFORMATION
Miflonide Breezhaler, 200 micrograms/inhalation dose,
inhalation powder in hard capsules
Miflonide Breezhaler, 400 micrograms/inhalation dose, inhalation powder in hard capsules
Budesonide
You should read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist.
Table of contents of the leaflet
- 1. What is Miflonide Breezhaler and what is it used for
- 2. Important information before using Miflonide Breezhaler
- 3. How to use Miflonide Breezhaler
- 4. Possible side effects
- 5. How to store Miflonide Breezhaler
- 6. Package contents and other information
1. What is Miflonide Breezhaler and what is it used for
Budesonide, the active substance of Miflonide Breezhaler, belongs to a group of medicines called corticosteroids and is used to treat asthma in adult patients and children and adolescents over 6 years of age, as well as to treat chronic obstructive pulmonary disease (COPD). Miflonide Breezhaler is used to reduce inflammatory changes in the lungs and help maintain airway patency, reducing asthma symptoms. Asthma is caused by inflammation of the small airways. Regular use of Miflonide Breezhaler helps prevent asthma attacks and makes breathing easier in chronic obstructive pulmonary disease. You should not stop using Miflonide Breezhaler regularly, even after your symptoms have disappeared. If you have any doubts about how Miflonide Breezhaler works or why it has been prescribed, you should consult a doctor.
2. Important information before using Miflonide Breezhaler
When not to use Miflonide Breezhaler
Warnings and precautions
Before starting to use Miflonide Breezhaler, you should discuss the following with your doctor:
You should immediately inform your doctor if you experience any of the following symptoms:
infections of the airways while using Miflonide Breezhaler (possible symptoms: increased cough, fever, discharge in the airways),
- breathing difficulties with wheezing or cough (paradoxical bronchospasm) after inhaling Miflonide Breezhaler,
- rash, itching, hives, breathing difficulties or swallowing, dizziness, swelling of the face or throat while using Miflonide Breezhaler,
- weight change, weakness, abdominal obesity, nausea, persistent diarrhea while using Miflonide Breezhaler,
- vision disturbances, including blurred vision while using Miflonide Breezhaler,
- sleep problems, depression or feeling anxious, restlessness, nervousness, overexcitement or irritability during treatment with Miflonide Breezhaler.
Other special warnings
- Do not use another inhaler.
- If your symptoms worsen, such as wheezing or shortness of breath, you should inform your doctor.
- Do not useMiflonide Breezhaler to treat acute shortness of breath attacks. In these cases, other medicines are used. If you have not been prescribed another medicine for this purpose, you should discuss it with your doctor.
- Do not suddenly stop using oral anti-inflammatory medicines (e.g., steroids taken orally). In patients who have been taking oral anti-inflammatory medicines for a long time, the doctor will gradually reduce the dose of these medicines when starting to use Miflonide Breezhaler. The doctor may recommend that the patient carry a warning card, as in the event of an accident, surgery, or severe infection, the patient may need to use higher doses of anti-inflammatory medicine.
- You should rinse your mouth with water after each use of Miflonide Breezhaler to reduce the risk of fungal infections of the mouth. Do not swallow the water used for rinsing.
- To alleviate asthma symptoms, the patient should have access to a short-acting bronchodilator (e.g., albuterol or salbutamol).
- The doctor may periodically perform an adrenal function test.
- It is recommended that patients always carry a card indicating that they have asthma.
- In the event of worsening asthma symptoms, the doctor may recommend increasing the dose of Miflonide Breezhaler or may also recommend the use of oral corticosteroids and/or an antibiotic in the event of an infection.
- In stressful situations, such as injury or surgery, the doctor may recommend the use of additional corticosteroids.
- Replacing systemic corticosteroids with inhaled corticosteroids may reveal the existence of allergic reactions, such as allergic rhinitis or hives, which were previously suppressed by oral corticosteroids. Patients may experience lethargy, muscle and joint pain, nausea, or vomiting. Allergic reactions can be treated with antihistamines or locally applied corticosteroids.
Allergic reactions may be treated with antihistamines or locally applied corticosteroids.
If the patient has been diagnosed with liver disease, they should inform their doctor before using Miflonide Breezhaler. The doctor will recommend an appropriate dose of the medicine.
- If the patient has been diagnosed with liver disease, they should inform their doctor before using Miflonide Breezhaler. The doctor will recommend an appropriate dose of the medicine.
Children and adolescents (over 6 years of age)
Miflonide Breezhaler should not be used in children under 6 years of age.
If a child is using a high dose of inhaled steroid for a long time, the doctor will regularly monitor the child's growth.
Miflonide Breezhaler and other medicines
You should tell your doctor about all medicines you are currently taking or have recently taken, as well as medicines that are available without a prescription. This is especially important for the following medicines:
- certain medicines used to treat infections (e.g., itraconazole, ketoconazole, clarithromycin, rifampicin),
- certain medicines used to treat HIV infections (e.g., ritonavir, nelfinavir, atazanavir, cobicistat),
- certain medicines used to treat heart rhythm disorders. If you are taking any of the above medicines, a dose change or other precautions may be necessary.
Pregnancy and breastfeeding
The medicine may be used during pregnancy only if, in the doctor's opinion, the benefit to the mother outweighs the potential risk to the fetus. If you are pregnant, think you may be pregnant, or plan to have a child, you should consult your doctor before using this medicine. You should discuss the risk of using the medicine during pregnancy with your doctor. You should use the smallest effective dose of budesonide necessary to maintain adequate control of asthma.
Miflonide Breezhaler is not recommended during breastfeeding. If you are breastfeeding, you should consult your doctor before using this medicine. The doctor will discuss the risk of using the medicine during breastfeeding with you.
Driving and using machines
It is unlikely that Miflonide Breezhaler will affect your ability to drive or use machines.
Important information about some of the ingredients of Miflonide Breezhaler
Miflonide Breezhaler contains lactose (milk sugar). If you have been diagnosed with intolerance to some sugars, e.g., lactose, you should contact your doctor before using Miflonide Breezhaler.
3. How to use Miflonide Breezhaler
Miflonide Breezhaler should always be used as directed by your doctor. If you have any doubts, you should consult a doctor. Do not exceed the recommended dose.
Miflonide Breezhaler is available in two doses. Your doctor has prescribed the optimal dose for you.
Dosage
Asthma
Adults: The dose is 200 to 400 micrograms of budesonide once or twice a day.
In the event of worsening asthma symptoms, when changing from oral corticosteroids to inhaled budesonide, or when reducing the dose of oral corticosteroids, the dose of budesonide may be increased to 1600 micrograms per day, administered in 2 to 4 inhalations.
Children over 6 years of age:The dose is 200 micrograms of budesonide once or twice a day. If necessary, the doctor may increase the dose of budesonide to 800 micrograms per day.
Children should use Miflonide Breezhaler under adult supervision.
Chronic obstructive pulmonary disease (COPD)
Adults: The dose is 200 to 400 micrograms of budesonide twice a day. If necessary, the doctor may increase the dose to 1600 micrograms per day.
If you have any doubts about using the medicine, you should consult a doctor or pharmacist.
Depending on the patient's response to treatment, the doctor may recommend a higher or lower dose of the medicine.
When to use Miflonide Breezhaler
Miflonide Breezhaler is best used at the same time every day.
If you feel that the effect of Miflonide Breezhaler is too strong or too weak, you should consult a doctor.
Instructions for use
You should carefully read the following instructions to learn how to use the Miflonide Breezhaler capsules with the Breezhaler inhaler.
You should only use the Miflonide Breezhaler capsules with the Breezhaler inhaler provided in the package.
- Do not use other inhalers.
- Do not swallow the capsules. The powder in the capsules is intended for inhalation.
- Remember that Miflonide Breezhaler is used only to prevent asthma attacks. To treat an asthma attack, you will need to use a rescue inhaler, usually used by the patient.
Product packaging:
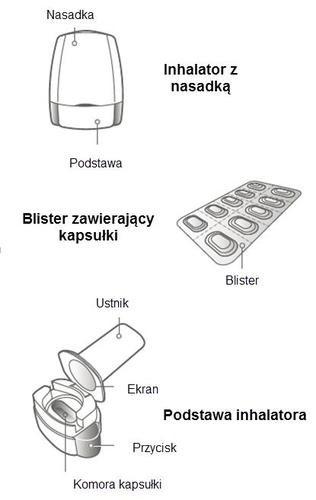
Each package of Miflonide Breezhaler contains:
- 1 Breezhaler inhaler
- 6 or 10 blisters containing Miflonide Breezhaler capsules for use with the inhaler. The Breezhaler inhaler is used to inhale the medicine contained in the Miflonide Breezhaler capsules. How to use the Breezhaler inhaler
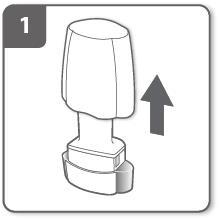
Remove the mouthpiece cover.
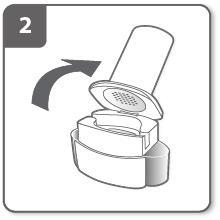
Open the inhaler:
Hold the base of the inhaler firmly and tilt the mouthpiece. This will open the inhaler.
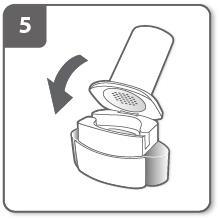
Prepare the capsule:
Take the capsule out of the blister with dry hands immediately before use. Do not swallow the capsule.
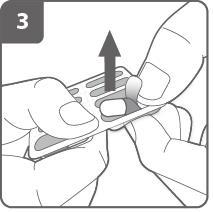
Insert the capsule:
Place the capsule in the capsule chamber.
Never insert the capsule directly into the mouthpiece.
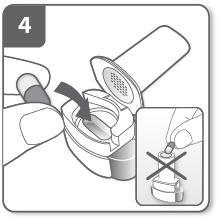
Close the inhaler:
Close the inhaler until you hear a "click".
Puncture the capsule:
- Hold the inhaler upright with the mouthpiece facing up.
- Puncture the capsule by firmly pressing the side buttons at the same time. This should only be done once.
- When puncturing the capsule, you should hear a "click".
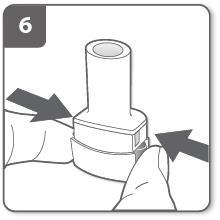
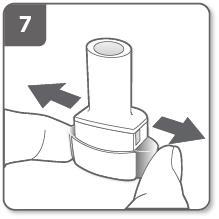
Release the side buttons completely.
Exhale:
Before putting the mouthpiece in your mouth, you should exhale fully.
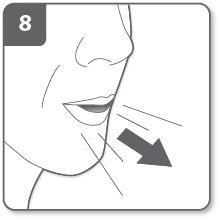
Do not blow into the mouthpiece.
Inhale the medicine:
To inhale the medicine deeply into your airways:
- Hold the inhaler as shown in the picture. The side buttons should be on the left and right. Do not press the side buttons.
- Put the mouthpiece in your mouth. Hold the mouthpiece tightly between your lips.
- Take a quick, but steady, breath in as deeply as possible.
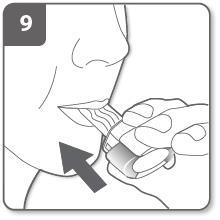
Note:
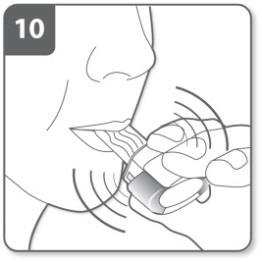
As you breathe in through the inhaler, the capsule will rotate in the chamber and you should hear a whistling sound. As the medicine moves into your lungs, you will feel a sweet taste.
Additional information
Occasionally, very small fragments of the capsule may pass through the filter and reach your mouth. If this happens, you may feel them on your tongue. Swallowing or inhaling these fragments is not harmful.
The likelihood of the capsule breaking is higher if the capsule is punctured more than once (see step 6).
If you do not hear a whistling sound:
The capsule may be jammed in the chamber. In this case, you should:
- Open the inhaler and carefully loosen the capsule by tapping the base of the inhaler. Do not press the side buttons.
- Repeat steps 8 and 9.
Hold your breath:
After inhaling the medicine, you should:
- Hold your breath for at least 5-10 seconds, or as long as it is comfortable, while removing the inhaler from your mouth.
- Then exhale.
- Open the inhaler to check if there is still powder in the capsule.
If there is still powder in the capsule:
- Close the inhaler.
- Repeat steps 8, 9, 10, and 11. Most people are able to empty the capsule in one or two inhalations.
Additional information
If the capsule is empty, it means that a sufficient amount of medicine has reached your lungs.
- Open the mouthpiece again and remove the empty capsule by tilting the inhaler so that the capsule falls out of the chamber. Dispose of the empty capsule in the trash.
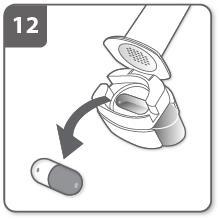
If your doctor has recommended that you take more than one capsule, you should repeat steps 3-12.
After finishing your dose
- Close the inhaler and put the mouthpiece cover back on. After using the medicine, rinse your mouth with water. Spit out the water used for rinsing. This will reduce the risk of fungal infections (thrush) in your mouth. Do not store the capsules in the Breezhaler inhaler.
How to clean the inhaler
The inhaler should never be washed with water. If the inhaler needs to be cleaned, you should wipe the inner and outer surface of the mouthpiece with a clean, dry, lint-free cloth to remove any powder residue. The inhaler must be dry.
Remember
- Do not swallow Miflonide Breezhaler capsules.
- Only use the Breezhaler inhaler provided in this package.
- The capsules must always be stored in blisters and removed from them immediately before use.
- Never insert a Miflonide Breezhaler capsule directly into the mouthpiece of the Breezhaler inhaler.
- Never press the side buttons more than once.
- Never blow into the Breezhaler inhaler mouthpiece.
- Always release the side buttons before inhaling.
- Never wash the Breezhaler inhaler with water. The inhaler must be dry. See "How to clean the inhaler".
- Never disassemble the Breezhaler inhaler.
- Always use a new Breezhaler inhaler provided with a new package of Miflonide Breezhaler. After finishing the package, you should always discard the inhaler.
- Do not store the capsules in the Breezhaler inhaler.
- Always store the Breezhaler inhaler and Miflonide Breezhaler capsules in a dry place.
How long to use Miflonide Breezhaler
It is essential to use Miflonide Breezhaler regularly as directed by your doctor. You should use Miflonide Breezhaler even when your asthma symptoms do not occur, as it helps prevent asthma attacks. If you have any doubts about how long to use Miflonide Breezhaler, you should consult a doctor.
Using more than the recommended dose of Miflonide Breezhaler
If you have taken a higher dose of the medicine than recommended, or if someone else has taken your dose by mistake, you should immediately contact a doctor or go to the hospital. You should take the package of the medicine with you. Appropriate treatment may be necessary.
It is essential to use the doses of the medicine as recommended by your doctor. Do not increase or decrease the dose without consulting a doctor.
Missing a dose of Miflonide Breezhaler
If you miss a dose, you should take the next dose at the usual time. DO NOTtake a double dose to make up for the missed dose.
Stopping use of Miflonide Breezhaler
Stopping the use of Miflonide Breezhaler may cause your asthma symptoms to worsen. DO NOTsuddenly stop using Miflonide Breezhaler unless your doctor has told you to do so.
If you have any further doubts about using the medicine, you should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Miflonide Breezhaler can cause side effects, although not everybody gets them.
Some rare side effects can be serious
- Breathing difficulties with wheezing or cough. You should stop using the medicine and contact your doctor immediately.
- Severe allergic reactions on the skin with rash, itching, hives, breathing difficulties or swallowing, dizziness, and (or) swelling of the face and throat; these may be symptoms of a severe allergic reaction.
- Extreme weakness, weight loss, nausea, persistent diarrhea; these may be symptoms of adrenal insufficiency.
- Weight gain, moon face, weakness, and (or) abdominal obesity; these may be symptoms of a hormonal disorder (Cushing's syndrome related to adrenal hyperfunction).
- Increased eye pressure (glaucoma). If you experience any of the above symptoms, you should contact your doctor immediately.
Other side effects
Common side effects
Side effects that may occur in up to 1 in 10 patients
- Fungal infections (candidiasis) of the mouth and throat. You should rinse your mouth with water after each dose to reduce the risk of fungal infections of the mouth and throat.
- Cough. If this symptom is severe, you should inform your doctor.
- Hoarseness
- Irritation of the throat
- Dysphonia (voice disorder, voice weakness, or change in voice pitch)
- Pneumonia (lung infection) in patients with COPD. You should tell your doctor if you experience any of the following symptoms while using budesonide; these may be symptoms of a lung infection:
- fever or chills
- increased production of mucus, change in mucus color
- worsening cough or increased breathing difficulties
Uncommon side effects
Side effects that may occur in up to 1 in 100 patients
- Blurred vision, cataract
- Muscle cramps
- Muscle tremors
- Depression
- Anxiety
Rare side effects
Side effects that may occur in up to 1 in 1000 patients
- Delayed growth in children and adolescents
- Weakening of bone structure
- Nervousness, changes in behavior (mainly in children)
- Immediate and delayed hypersensitivity reactions, including rash, contact dermatitis, hives, angioedema, itching, and severe allergic reactions
- Easy bruising
If you experience any of the above side effects to a significant degree, you should inform your doctor.
Other side effects may occur, but their frequency cannot be estimated from the available data
- Sleep problems, excessive psychomotor activity, aggression. The occurrence of these side effects is more likely in children. If you experience any of the above side effects, you should inform your doctor.
If your symptoms worsen or if you experience side effects not listed in this leaflet, you should tell your doctor or pharmacist.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel. +48 22 49 21 301, fax: +48 22 49 21 309, e-mail: [email protected]. By reporting side effects, you can help provide more information on the safety of the medicine.
Side effects can also be reported to the marketing authorization holder.
5. How to store Miflonide Breezhaler
- Store the medicine at a temperature below 25°C, in a dry place.
- The medicine should be stored out of sight and reach of children.
- Do not use Miflonide Breezhaler if the package is damaged.
- Do not use Miflonide Breezhaler after the expiry date stated on the package. The expiry date refers to the last day of the month stated.
- Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Miflonide Breezhaler contains
- The active substance of Miflonide Breezhaler is budesonide. One capsule (i.e., one inhalation dose) of Miflonide Breezhaler contains 200 or 400 micrograms of budesonide.
- The other ingredients of the medicine are: lactose monohydrate. The ingredients of the capsule shell are: gelatin, purified water, iron oxide red (E 172), titanium dioxide (E171), and iron oxide black (E 172) (only for the 400 microgram dose) and cosine red (E124) (only for the 400 microgram dose). The ingredients of the ink used for printing on the capsules are: shellac (E 904), propylene glycol (E 1520), concentrated ammonia (E 527), iron oxide black (E 172), and potassium hydroxide (E 525).
What Miflonide Breezhaler looks like and package contents
Miflonide Breezhaler is a pinkish-transparent powder for inhalation in capsules. The powder in the capsule is intended for inhalation into the lungs using an inhaler called Breezhaler.
The package contains 60 or 100 capsules in blisters and an inhaler.
Marketing authorization holder
Novartis Poland Sp. z o.o.
ul. Marynarska 15
02-674 Warsaw
Phone: +48 22 375 48 88
Manufacturer/importer:
Novartis Poland Sp. z o.o.
ul. Marynarska 15
02-674 Warsaw
Novartis Pharma GmbH
Roonstrasse 25,
90429 Nürnberg
Germany
Novartis Farmacéutica S.A.
Gran Vía de les Corts Catalanes, 764,
08013 Barcelona
Spain
Date of last update of the leaflet:06/2021
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterNovartis Farmaceutica S.A. Novartis Pharma GmbH Novartis Poland Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Miflonide BreezhalerDosage form: Suspension, 0.125 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.25 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.5 mg/mlActive substance: budesonidePrescription required
Alternatives to Miflonide Breezhaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Miflonide Breezhaler in Ukraine
Alternative to Miflonide Breezhaler in Spain
Online doctors for Miflonide Breezhaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Miflonide Breezhaler – subject to medical assessment and local rules.