
Metoclopramidi hidrohloridum Noridem
Ask a doctor about a prescription for Metoclopramidi hidrohloridum Noridem

How to use Metoclopramidi hidrohloridum Noridem
PATIENT INFORMATION LEAFLET
Leaflet attached to the packaging: information for the user
Metoclopramide hydrochloride Noridem, 5 mg/mL, solution for injection
Metoclopramide hydrochloride anhydrous
You should carefully read the contents of this leaflet before taking the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any further questions, please ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Metoclopramide hydrochloride Noridem and what is it used for
- 2. Important information before taking Metoclopramide hydrochloride Noridem
- 3. How to take Metoclopramide hydrochloride Noridem
- 4. Possible side effects
- 5. How to store Metoclopramide hydrochloride Noridem
- 6. Contents of the packaging and other information
1. What is Metoclopramide hydrochloride Noridem and what is it used for
Metoclopramide hydrochloride Noridem is an antiemetic medicine. It contains the active substance called "metoclopramide". The medicine works on the part of the brain that prevents nausea and vomiting. Metoclopramide hydrochloride Noridem is used in adults:
- to prevent nausea and vomiting that may occur after surgery
- to treat nausea and vomiting, including nausea and vomiting associated with migraine
- to prevent nausea and vomiting associated with radiotherapy
Metoclopramide hydrochloride Noridem is indicated for use in children and adolescents(aged 1-18 years), only if other treatment methods have proven ineffective or cannot be used:
- to prevent delayed nausea and vomiting that may occur after chemotherapy
- to treat nausea and vomiting that occur after surgical procedures
2. Important information before taking Metoclopramide hydrochloride Noridem
When not to use Metoclopramide hydrochloride Noridem:
if the patient is allergic to metoclopramide or any of the other ingredients of this medicine (listed in section 6).
in case of bleeding, obstruction, or perforation of the stomach or intestines.
if the patient has a rare adrenal gland tumor located near the kidney (pheochromocytoma).
if the patient has or has had involuntary muscle contractions (tardive dyskinesia) during treatment with medicines.
if the patient has epilepsy
if the patient has Parkinson's disease
if the patient is taking levodopa (a medicine for Parkinson's disease) or dopamine agonists (see below "Other medicines and Metoclopramide hydrochloride Noridem")
if the patient has ever had abnormal blood pigment levels (methemoglobinemia) or a deficiency of the enzyme NADH cytochrome b5 reductase.
Metoclopramide hydrochloride Noridem should not be used in children under 1 year of age (see below "Children and adolescents").
Warnings and precautions
Before starting treatment with Metoclopramide hydrochloride Noridem, you should tell your doctor, pharmacist, or nurse if:
- you have had heart rhythm disorders (prolonged QT interval) or other heart problems in the past
- you have electrolyte disturbances, such as potassium, sodium, or magnesium levels
- you are taking other medicines that affect the heart
- you have neurological disorders (related to the brain)
- you have kidney or liver problems. The dose may need to be reduced (see section 3).
Your doctor may order a blood test to check the blood pigment levels. If the levels are abnormal (methemoglobinemia), the medicine should be stopped immediately and permanently.
Children and adolescents
Uncontrolled movements (extrapyramidal symptoms) may occur in children and adolescents. This medicine should not be used in children under 1 year of age due to the increased risk of uncontrolled movements (see "When not to use Metoclopramide hydrochloride Noridem").
Other medicines and Metoclopramide hydrochloride Noridem
You should tell your doctor, pharmacist, or nurse about all the medicines you are taking or have recently taken, as well as any medicines you plan to take. This is important because some medicines may affect the action of Metoclopramide hydrochloride Noridem or Metoclopramide hydrochloride Noridem may affect the action of other medicines. In particular, you should inform your doctor about the use of the following medicines:
levodopa or other medicines used to treat Parkinson's disease (see "When not to use Metoclopramide hydrochloride Noridem")
anticholinergic medicines (used to relieve stomach cramps)
opioid derivatives (used to treat severe pain)
sedatives
medicines used to treat mental health problems
digoxin (a medicine used to treat heart failure)
cyclosporin (a medicine used to treat certain immune system disorders)
mivacurium and suxamethonium (medicines used to relax muscles)
fluoxetine and paroxetine (medicines used to treat depression)
Using Metoclopramide hydrochloride Noridem with alcohol
During treatment with metoclopramide, you should not drink alcohol, as it may enhance the sedative effect of Metoclopramide hydrochloride Noridem.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or plan to become pregnant, you should consult your doctor or pharmacist before taking this medicine. If necessary, Metoclopramide hydrochloride Noridem may be used during pregnancy. Your doctor will decide whether you should take the medicine.
It is not recommended to use Metoclopramide hydrochloride Noridem during breastfeeding, as metoclopramide passes into breast milk and may affect the baby.
Driving and using machines
You may feel drowsy, dizzy, or experience uncontrolled movements, perform sudden or twisting movements, and unusual muscle tension causing body distortion after taking Metoclopramide hydrochloride Noridem. This may cause vision disturbances and affect your ability to drive or operate machinery.
Metoclopramide hydrochloride Noridem contains sodium
Each mL contains 3.35 mg (0.1455 mmol) of sodium.
The medicine contains less than 1 mmol (23 mg) of sodium per ampoule, which means that the medicine is considered "sodium-free".
3. How to take Metoclopramide hydrochloride Noridem
The medicine will usually be administered by a doctor or nurse. The medicine will be administered as a slow injection into a vein (over at least 3 minutes) or as an intramuscular injection.
Use in adults
Treatment of nausea and vomiting, including nausea and vomiting associated with migraine, and prevention of nausea and vomiting associated with radiotherapy: the recommended single dose is 10 mg. The dose can be repeated up to three times a day.
The maximum recommended daily dose is 30 mg or 0.5 mg/kg body weight.
Prevention of nausea and vomiting after surgery: the recommended single dose is 10 mg.
Use in children and adolescents aged 1-18 years (all indications)
The recommended dose is 0.1 to 0.15 mg/kg body weight, repeated up to three times a day, administered as a slow injection into a vein.
The maximum daily dose is 0.5 mg/kg body weight.
Dosing table
Age
Body weight
Dose
Frequency
1-3 years
10-14 kg
1 mg
Up to 3 times a day
3-5 years
15-19 kg
2 mg
Up to 3 times a day
5-9 years
20-29 kg
2.5 mg
Up to 3 times a day
9-15 years
30-60 kg
5 mg
Up to 3 times a day
15-18 years
Over 60 kg
10 mg
Up to 3 times a day
Treatment should not last longer than 48 hours in the case of treatment of nausea and vomiting after surgery.
Treatment should not last longer than 5 days in the case of prevention of delayed nausea and vomiting after chemotherapy.
Use in the elderly
A dose reduction may be necessary depending on kidney and liver function and overall health.
Other pharmaceutical forms may be more suitable for this patient group.
Use in adults with renal impairment
You should inform your doctor about kidney problems. In patients with severe liver impairment, the dose should be reduced.
Other pharmaceutical forms may be more suitable for this patient group.
Use in adults with hepatic impairment
You should inform your doctor about liver problems. In patients with severe liver impairment, the dose should be reduced.
Other pharmaceutical forms may be more suitable for this patient group.
Use in children under 1 year of age
Metoclopramide should not be used in children under 1 year of age (see section 2).
Taking a higher dose of Metoclopramide hydrochloride Noridem than recommended
You should immediately contact your doctor or pharmacist. The patient may experience uncontrolled movements (extrapyramidal symptoms), drowsiness, consciousness disorders, disorientation, hallucinations, and heart problems. If necessary, the doctor will order appropriate symptomatic treatment.
Missing a dose of Metoclopramide hydrochloride Noridem
You should not take a double dose to make up for a missed dose.
If you have any further questions about the use of this medicine, please ask your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following side effects, you should stop taking this medicine and immediately inform your doctor, pharmacist, or nurse:
Uncontrolled movements (often in the head and neck area). These can occur in children and young adults, especially when using high doses. These symptoms usually occur at the beginning of treatment and can occur even after a single dose. Proper treatment will stop the movements.
High fever, high blood pressure, seizures, sweating, salivation. These may be symptoms of a disorder called malignant neuroleptic syndrome.
Skin itching or rash, facial, lip, or throat swelling, breathing difficulties. These may be symptoms of a potentially severe allergic reaction.
Very common(may affect more than 1 in 10 people)
- •drowsiness
Common(may affect up to 1 in 10 people)
- •depression
- uncontrolled movements, such as tics, tremors, twisting, or muscle stiffness
- symptoms similar to those of Parkinson's disease (stiffness, tremors)
- restlessness
- low blood pressure (especially after intravenous administration)
- diarrhea
- weakness. Uncommon(may affect up to 1 in 100 people)
- •elevated levels of a hormone called prolactin in the blood, which may cause: milk production in men, and in non-breastfeeding women, irregular periods
- hallucinations
- reduced level of consciousness
- slow heart rate (especially after intravenous administration)
- allergic reactions
- vision problems and involuntary eye movements
Rare(may affect up to 1 in 1000 people)
confusion
seizures (especially in patients with epilepsy)
Frequency not known(frequency cannot be estimated from the available data)
- •abnormal blood pigment levels, which may cause skin color changes
- abnormal breast growth (gynecomastia)
- involuntary muscle contractions after long-term treatment, especially in elderly patients
- high fever, high blood pressure, seizures, sweating, salivation. These may be symptoms of a disorder called malignant neuroleptic syndrome
- heart rhythm changes, which may be visible on an ECG
- cardiac arrest (especially after injection)
- shock (severe drop in blood pressure) (especially after injection)
- loss of consciousness (especially after intravenous administration)
- allergic reaction, which may be severe (especially after intravenous administration)
- very high blood pressure
- suicidal thoughts.
If you experience any side effects, please inform your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor or nurse. Side effects can also be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products,
Jerozolimskie Avenue 181C
02-222 Warsaw,
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl .
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Metoclopramide hydrochloride Noridem
The medicine should be stored out of sight and reach of children.
Ampoules should be stored in a protective bag and outer packaging to protect from light.
The medicine does not require any special storage conditions in terms of temperature.
After first opening:
Use within 2 months if the ampoules are stored without the protective bag.
After mixing/dilution: Chemical and physical stability after mixing with 0.9% sodium chloride, 5% dextrose, Ringer's solution with lactate, and 4% dextrose in 0.18% sodium chloride has been demonstrated for 48 hours at 15-25°C in artificial light and for 48 hours at 5 (±3) °C, at a concentration of Metoclopramide hydrochloride Noridem 0.1 mg/mL.
From a microbiological point of view, unless the method of opening precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user.
Do not use this medicine after the expiry date stated on the protective bag and carton after "EXP". The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Metoclopramide hydrochloride Noridem contains
- The active substance is metoclopramide hydrochloride anhydrous (in the form of metoclopramide hydrochloride monohydrate). Each mL of solution contains 5.27 mg of metoclopramide hydrochloride monohydrate, which corresponds to 5 mg of metoclopramide hydrochloride anhydrous.
- The other ingredients are sodium chloride, sodium hydroxide, and/or hydrochloric acid, and water for injections.
What Metoclopramide hydrochloride Noridem looks like and contents of the pack
Metoclopramide hydrochloride Noridem, 5 mg/mL, solution for injection is a clear, colorless solution for injection.
PP ampoules containing 2 mL of solution, packaged in cartons of 5, 10 (2 x 5), 20 (4 x 5), 50 (10 x 5), or 60 (12 x 5) ampoules.
Each 5 ampoules is wrapped in a protective bag.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:Noridem Enterprises Ltd, Makariou & Evagorou 1, Mitsi Building 3, Office 115, 1065 Nicosia, Cyprus.
Manufacturer:DEMO S.A., PHARMACEUTICAL INDUSTRY, 21st km National Road Athens-Lamia, 14568 Krioneri, Attiki, Greece. T.: +302108161802, F.: +302108161587
This medicine is authorized in the Member States of the European Economic Area under the following names:
Date of last revision of the leaflet:
<--------------------------------------------------------------------------------------------------------------------------
--->
| Cyprus | PRIBEKINET 5 mg / mL Solution for injection |
| Czech Republic | Metoclopramide Noridem |
| Germany | Metoclopramidhydrochlorid Noridem 5 mg/ml Injektionslösung |
| Greece | PRIBEKINET 5 mg / mL Ενέσιμο Διάλυμα |
| France | METOCLOPRAMIDE NORIDEM 10 mg/2 mL, solution injectable |
| Hungary | Metoklopramid-hidroklorid Noridem 5 mg/ml oldatos injekció |
| Poland | Metoclopramidi hydrochloridum Noridem |
| Slovakia | Metoclopramide Noridem 5 mg/ml injekčný roztok |
Information intended for healthcare professionals only:
Preparation and disposal
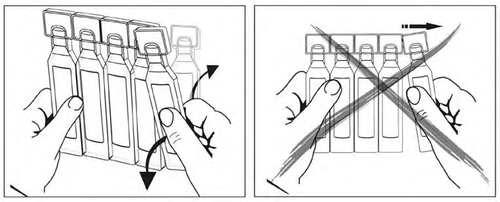
Incompatibilities
Due to the lack of compatibility studies, this medicinal product should not be mixed with other medicinal products, except for the following solutions:
- 0.9% sodium chloride solution,
- 5% dextrose solution,
- Ringer's solution with lactate,
- 4% dextrose in 0.18% sodium chloride solution to a final concentration of Metoclopramide hydrochloride Noridem 0.1 mg/mL.
Dosage and administration
All indications (adults)
Information on dosing can be found in section 3 of the package leaflet.
The duration of treatment with injections should be as short as possible, and switching to oral or rectal treatment should be done as soon as possible.
Administration frequency:
A minimum interval of 6 hours should be maintained between two administrations, even in case of vomiting or rejection of the dose.
Special patient groups
Elderly patients:
In elderly patients, the dose should be reduced depending on kidney and liver function and overall health.
Renal impairment:
In patients with severe renal impairment (creatinine clearance <15 ml min), the daily dose should be reduced by 75%.
In patients with moderate or severe renal impairment (creatinine clearance 15-60 mL/min), the daily dose should be reduced by 50%.
Hepatic impairment:
In patients with severe hepatic impairment, the dose should be reduced by 50%.
Other pharmaceutical forms may be more suitable for this population.
Children and adolescents:
Metoclopramide is contraindicated in children under 1 year of age.
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local regulations.
Overdose
Symptoms
Extrapyramidal disorders, drowsiness, decreased level of consciousness, confusion, hallucinations, and cardiac and respiratory arrest may occur.
Treatment
In case of extrapyramidal symptoms related or not related to overdose, treatment is only symptomatic (in children, benzodiazepines and/or anticholinergic medicines used in Parkinson's disease in adults).
Depending on the clinical condition, symptomatic treatment should be applied, and cardiovascular and respiratory functions should be constantly monitored.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterDEMO S.A. Pharmaceutical Industry
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Metoclopramidi hidrohloridum NoridemDosage form: Solution, 5 mg/mlActive substance: metoclopramidePrescription requiredDosage form: Solution, 5 mg/mlActive substance: metoclopramideManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A.Prescription requiredDosage form: Tablets, 10 mgActive substance: metoclopramideManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A.Prescription required
Alternatives to Metoclopramidi hidrohloridum Noridem in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Metoclopramidi hidrohloridum Noridem in Spain
Alternative to Metoclopramidi hidrohloridum Noridem in Ukraine
Online doctors for Metoclopramidi hidrohloridum Noridem
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Metoclopramidi hidrohloridum Noridem – subject to medical assessment and local rules.














