How to use Metex Pen
Leaflet accompanying the packaging: information for the user
Metex PEN, 7.5 mg, solution for injection in a pre-filled pen
Metex PEN, 10 mg, solution for injection in a pre-filled pen
Metex PEN, 12.5 mg, solution for injection in a pre-filled pen
Metex PEN, 15 mg, solution for injection in a pre-filled pen
Metex PEN, 17.5 mg, solution for injection in a pre-filled pen
Metex PEN, 20 mg, solution for injection in a pre-filled pen
Metex PEN, 22.5 mg, solution for injection in a pre-filled pen
Metex PEN, 25 mg, solution for injection in a pre-filled pen
Metex PEN, 27.5 mg, solution for injection in a pre-filled pen
Metex PEN, 30 mg, solution for injection in a pre-filled pen
Methotrexate
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if you need to.
- If you have any doubts, you should consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Metex PEN and what is it used for
- 2. Important information before using Metex PEN
- 3. How to use Metex PEN
- 4. Possible side effects
- 5. How to store Metex PEN
- 6. Contents of the packaging and other information
1. What is Metex PEN and what is it used for
Indications for the use of the Metex PEN medicinal product
- active rheumatoid arthritis in adult patients, polyarticular forms of severe, active juvenile idiopathic arthritis, if the response to non-steroidal anti-inflammatory drugs (NSAIDs) is insufficient,
- moderate and severe psoriasis in adult patients, and severe psoriatic arthritis in adults,
- Crohn's disease with a mild to moderate course in adult patients, when appropriate treatment with other medicines is not possible.
Rheumatoid arthritis(RA) is a chronic disease classified as collagenosis, characterized by inflammation of the synovial membranes lining the joints. The synovial membranes produce fluid that acts as a lubricant for many joints. The inflammatory state causes thickening of the membranes and swelling of the joints.
Juvenile arthritisoccurs in children and adolescents under the age of 16. The fact that 5 or more joints are affected by the disease within the first 6 months of the disease indicates a polyarticular form.
Psoriasisis a common chronic skin disease, characterized by red patches covered with thick, dry, silvery, tightly adhering scales.
Psoriatic arthritisrefers to joint inflammation, especially of the fingers and toes, with psoriatic changes on the skin and nails.
Metex PEN modifies and slows down the progression of the disease.
Crohn's disease is a type of inflammatory bowel disease that can affect any part of the digestive tract, causing symptoms such as abdominal pain, diarrhea, vomiting, or weight loss.
2. Important information before using Metex PEN
When not to use Metex PEN
- if the patient is allergic to methotrexate or any of the other ingredients of this medicine (listed in section 6);
- if the patient has liver disease, severe kidney disease, or blood disorders;
- if the patient regularly consumes large amounts of alcohol;
- if the patient has severe infections, such as tuberculosis, HIV infection, or other immune system disorders;
- if the patient has ulcers of the mucous membrane of the mouth, stomach ulcers, or intestinal ulcers;
- if the patient is pregnant or breastfeeding (see "Pregnancy, breastfeeding, and fertility");
- if the patient is receiving a vaccine containing live microorganisms at the same time.
Warnings and precautions
Before starting treatment with Metex PEN, you should discuss the following with your doctor or pharmacist:
- if the patient is elderly, frail, or in poor general health;
- if the patient has liver function disorders;
- if the patient has been diagnosed with dehydration (lack of water in the body);
- if the patient has diabetes and is taking insulin.
Special precautions for the use of Metex PEN
Methotrexate temporarily disrupts the production of sperm and egg cells; in most cases, this effect disappears. Methotrexate may cause miscarriage and severe birth defects. Women should avoid becoming pregnant while taking methotrexate and for at least 6 months after the end of treatment. Men should avoid fertilizing their partner while taking methotrexate and for at least 3 months after the end of treatment. See also "Pregnancy, breastfeeding, and fertility".
Recommended tests and precautions
Severe side effects may occur even after the use of small doses of methotrexate. To detect them in time, the doctor must perform regular check-ups and laboratory tests.
Before starting treatment
Before starting treatment, a blood test will be performed to check if the patient has a sufficient number of blood cells. Blood tests will also be performed to assess liver function and check for hepatitis. Additionally, the level of albumin (a blood protein) in the serum and kidney function will be checked, and an assessment for hepatitis (liver infection) will be performed. The doctor may also decide to perform other liver tests, which may include liver imaging tests or a liver biopsy to assess the liver more accurately. Additionally, the doctor may check for tuberculosis and may perform a chest X-ray or lung function test.
During treatment
The doctor may perform the following tests:
- examination of the mouth and throat for mucous membrane changes, such as inflammation or ulcers;
- blood tests/blood cell counts and assessment of methotrexate levels in the blood serum;
- blood test to monitor liver function;
- imaging tests to monitor liver condition;
- liver biopsy to assess the liver more accurately;
- blood test to monitor kidney function;
- respiratory system check-up and, if necessary, lung function test.
It is very important for the patient to attend these scheduled check-ups.
If the results of any of these tests show abnormalities, the doctor will adjust the treatment accordingly.
Elderly patients
Elderly patients treated with methotrexate should be under close medical supervision to detect possible side effects as soon as possible.
Age-related liver and kidney function disorders and low folate levels in the elderly require the use of relatively low doses of methotrexate.
Other precautions
During treatment with methotrexate, cases of acute bleeding from the lungs have been reported in patients with underlying rheumatologic disease. If the patient experiences hemoptysis (coughing up blood), they should immediately consult their doctor.
Methotrexate may affect the immune system, the effectiveness of vaccines, and the results of immunological tests. It may lead to the reactivation of latent chronic diseases (e.g., shingles, tuberculosis, hepatitis B or C). During treatment with Metex PEN
do not use live microorganism vaccines.
Methotrexate may increase skin sensitivity to sunlight. You should avoid intense sun exposure and not use a sunbed or sunlamp without consulting your doctor. To protect your skin from intense sunlight, wear appropriate clothing or use a sunscreen with a high protection factor.
During methotrexate treatment, radiation-induced skin inflammation and sunburn (recall reaction) may occur. Exposure to UV radiation during methotrexate treatment may exacerbate psoriatic lesions.
Lymph node enlargement (lymphoma) may occur. In such cases, treatment should be discontinued.
Diarrhea may be a sign of toxic effects of Metex PEN and requires treatment discontinuation. If the patient experiences diarrhea, they should tell their doctor.
In patients with cancer receiving methotrexate treatment, cases of certain brain function disorders (encephalopathy/leukoencephalopathy) have been reported. It cannot be excluded that such side effects may occur in the treatment of other diseases.
If the patient, their partner, or caregiver notices new onset or worsening of neurological symptoms, including general muscle weakness, vision disturbances, changes in thinking, memory, and orientation leading to disorientation and personality changes, they should immediately consult their doctor, as these may be symptoms of a very rare, severe brain infection called progressive multifocal leukoencephalopathy (PML).
Metex PEN and other medicines
Tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take in the future.
The concurrent use of certain medicines may affect the action of Metex PEN:
- antibiotics, e.g., tetracyclines, chloramphenicol, non-absorbable broad-spectrum antibiotics, penicillins, glycopeptides, sulfonamides, ciprofloxacin, and cefalotin (medicines used to prevent and treat certain infections);
- non-steroidal anti-inflammatory drugsor salicylates(pain-relieving and/or anti-inflammatory medicines, e.g., acetylsalicylic acid, diclofenac, and ibuprofen or pyrazole);
- metamizole (synonyms: novaminsulfone and dipyrone) (strong pain-relieving and/or antipyretic medicine);
- probenecid(used to treat gout);
- weak organic acids such as loop diuretics(diuretic medicines);
- medicines that may harm bone marrow function, e.g., trimethoprim-sulfamethoxazole (bactericidal substance) or pyrimethamine;
- other medicines used to treat rheumatoid arthritis, e.g., leflunomide, sulfasalazine, and azathioprine;
- cyclosporine (immunosuppressive medicine);
- mercaptopurine (cytostatic medicine);
- retinoids (medicines for psoriasisand other skin diseases);
- theophylline (medicine for asthmaand other lung diseases);
- certain stomach medicines, e.g., omeprazole and pantoprazole;
- blood glucose-lowering medicines (hypoglycemic medicines).
Vitamin products containing folateshould only be used if prescribed by a doctor, as they may reduce the effect of methotrexate.
Avoid live microorganism vaccines.
Metex PEN with food, drink, and alcohol
While using Metex PEN, you should avoid consuming alcohol and large amounts of coffee, caffeinated beverages, and black tea.
Pregnancy, breastfeeding, and fertility
Pregnancy
Do not use Metex PEN if you are pregnant or trying to become pregnant. Methotrexate may cause birth defects, harm the unborn child, or cause miscarriage. This is associated with developmental abnormalities of the skull, face, heart, and blood vessels, brain, and limbs. Therefore, it is very important that pregnant women or those planning to become pregnant do not take methotrexate. If the woman is of childbearing age, it must be definitively confirmed that she is not pregnant before starting treatment, by taking appropriate measures, e.g., performing a pregnancy test.
The patient should avoid becoming pregnant while taking methotrexate and for at least 6 months after the end of treatment, using reliable contraceptive methods throughout this time (see also "Warnings and precautions").
If the patient becomes pregnant during treatment or suspects that she may be pregnant, she should consult her doctor as soon as possible. The patient should receive advice on the possible harmful effects of treatment on the child.
If the patient plans to become pregnant, she should consult her attending doctor, who may refer her to a specialist for advice before planned treatment begins.
Breastfeeding
Breastfeeding should be discontinued before starting and during treatment with Metex PEN.
Male fertility
Available evidence does not indicate an increased risk of birth defects or miscarriages after the father has taken methotrexate at a dose below 30 mg/week. However, the risk cannot be entirely excluded. Methotrexate may be genotoxic, meaning it may cause genetic mutations. Methotrexate may affect sperm and cause birth defects. Therefore, the patient should avoid fertilizing their partner and should not donate sperm while taking methotrexate and for at least 3 months after the end of treatment.
Driving and using machines
While using Metex PEN, side effects such as fatigue and dizziness may occur. In connection with this, the ability to drive vehicles or operate machines may be impaired in some cases. If you experience drowsiness or fatigue, do not drive vehicles or operate machines.
Metex PEN contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per dose, which means the medicine is considered "sodium-free".
3. How to use Metex PEN
Important warning regarding Metex PEN dosing (methotrexate):
In the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, psoriatic arthritis, and Crohn's disease, Metex PEN should be used only once a week. Using more Metex PEN (methotrexate) than prescribed may be fatal. You should read section 3 of this leaflet very carefully. If you have any questions, consult your doctor or pharmacist before taking the medicine.
This medicine should always be used exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist.
The doctor will decide on the dosage, which is tailored to the individual patient.
The effects of treatment are usually visible only after 4-8 weeks.
Metex PEN is administered by subcutaneous injection by or under the supervision of a doctor or a qualified healthcare professional only once a week.The patient and doctor should agree on the day of the week when the injection will be administered.
Use in children and adolescents
The doctor will decide on the appropriate dose for children and adolescents with polyarticular juvenile idiopathic arthritis.
Metex PEN is not recommended for use in children under 3 years of agedue to insufficient experience in this age group.
Method and time of administration
Metex PEN is injected once a week!
The duration of treatment is determined by the attending doctor. Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, psoriatic arthritis, and Crohn's disease with Metex PEN is long-term.
Initially, Metex PEN may be administered by medical personnel. However, the doctor may decide that the patient is able to learn self-injection of Metex PEN. The patient will be properly trained in this regard. Never attempt to self-inject the medicine without prior training.
The "Instructions for use" at the end of the leaflet provide guidance on how to properly use Metex PEN.
Remember to use the entire contents.
The method of preparing the medicine for use and disposing of the medicine and the pre-filled pen must comply with local requirements. Pregnant healthcare workers should not prepare or administer the Metex PEN medicinal product.
Methotrexate must not come into contact with skin or mucous membranes. In case of contact, the contaminated area should be rinsed immediately with plenty of water.
Using a higher dose of Metex PEN than recommended
If the patient has used a higher dose of Metex PEN than recommended, they should immediately consult their doctor.
Missing a dose of Metex PEN
Do not take a double dose to make up for a missed dose.
Stopping treatment with Metex PEN
If treatment with Metex PEN is discontinued, the patient should immediately inform their doctor.
If the patient feels that the effect of Metex PEN is too strong or too weak, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Metex PEN can cause side effects, although not everybody gets them.
The frequency and severity of side effects depend on the dose and frequency of administration of the medicine. Since severe side effects may occur even after the use of small doses of methotrexate, regular medical check-ups are necessary. Therefore, the doctor should perform tests to rule out abnormalitiesin blood parameters (e.g., low white blood cell count, platelet count, lymphoma) and changes in the liver and kidneys.
Tell your doctor immediatelyif you experience any of the following symptoms, as they may indicate severe, potentially life-threatening side effects that require urgent treatment:
- persistent dry cough without expectoration, shortness of breath, and fever; these may be symptoms of pneumonia [frequent]
- hemoptysis (coughing up blood); this may be a sign of pulmonary bleeding [unknown frequency]
- symptoms of liver damage, such as yellowing of the skin and whites of the eyes; methotrexate may cause chronic liver damage (liver cirrhosis), scarring of liver tissue (liver fibrosis), fatty liver degeneration [all uncommon], acute liver inflammation (acute hepatitis) [rare], and liver failure [very rare]
- allergic reaction symptoms, such as skin rash, including itchy red skin, swelling of hands, feet, ankles, face, lips, mouth, or throat (which may cause swallowing or breathing difficulties) and the patient may feel like they are about to faint;these may be symptoms of severe allergic reactions or anaphylactic shock [rare]
- symptoms of kidney damage, such as swelling of hands, ankles, or feet, or changes in urination frequency or volume (oliguria) or absence of urine (anuria);these may be symptoms of kidney failure [rare]
- infection symptoms, such as fever, chills, pain, sore throat;methotrexate may increase the risk of infections. Severe infections, such as a certain type of pneumonia (Pneumocystis jirovecii pneumonia) and sepsis, may occur [rare]
- symptoms such as weakness on one side of the body (stroke) or pain, swelling, redness, and unusual warmth in one leg (deep vein thrombosis); this may occur if a blood clot blocks a blood vessel(thromboembolic event) [rare]
- fever and severe deterioration of general health or sudden fever, which is accompanied by sore throat or mouth and urinary disorders;methotrexate may cause severe reduction of certain white blood cells (agranulocytosis) and severe bone marrow suppression [very rare]
- unexpected bleeding, such as bleeding from the gums, blood in the urine, vomit, or appearance of blood bruises;these may be symptoms of significant reduction of platelet count in severe bone marrow suppression [very rare]
- symptoms such as severe headache with accompanying fever, stiff neck, nausea, vomiting, disorientation, and sensitivity to lightmay indicate meningitis (acute aseptic meningitis) [very rare]
- in patients with cancer receiving methotrexate treatment, cases of certain brain function disorders (encephalopathy/leukoencephalopathy) have been reported. It cannot be excluded that such side effects may occur in the treatment of other diseases. Symptoms of this type of brain function disorder include altered mental state, movement disorders (ataxia), vision disturbances, and memory disorders[unknown frequency]
- severe skin rash or blisters on the skin (which may also occur in the mouth, eyes, and genitals);these may be symptoms of Stevens-Johnson syndrome or toxic epidermal necrolysis (Lyell's syndrome) [very rare]
The following side effects may also occur:
Very common:may occur in more than 1 in 10 people
- mouth inflammation, nausea, vomiting, decreased appetite, abdominal pain,
- abnormal liver test results (AST, ALT, bilirubin, alkaline phosphatase).
Common:may occur in up to 1 in 10 people
- mouth ulcers, diarrhea,
- skin rash, redness, itching,
- headache, fatigue, drowsiness,
- decreased production of blood cells, leading to a decrease in white blood cells and/or red blood cells and/or platelets.
Uncommon:may occur in up to 1 in 100 people
- pharyngitis,
- enteritis, vomiting, pancreatitis, cholecystitis, black or tarry stools, gastrointestinal bleeding, and ulcers,
- reactions similar to sunburn due to increased skin sensitivity to sunlight, hair loss, increased number of rheumatoid nodules, skin ulcers, shingles, vasculitis, rash similar to herpes, hives,
- diabetes onset,
- dizziness, confusion, depression,
- decreased serum albumin levels,
- decreased production of all blood cells and platelets,
- cystitis, urethritis, impaired renal function, urinary disorders,
- arthralgia, myalgia, decreased bone mass.
Rare:may occur in up to 1 in 1,000 people
- gingivitis,
- increased pigmentation of the skin, acne, blue spots on the skin due to bleeding from blood vessels (petechiae, ecchymoses), allergic vasculitis,
- decreased levels of antibodies in the blood,
- infection (including latent chronic infection), conjunctivitis,
- mood changes (mood swings),
- vision disturbances,
- pericarditis, pericardial effusion, constrictive pericarditis,
- low blood pressure,
- pulmonary fibrosis, dyspnea, and asthma, pleural effusion,
- stress fractures,
- electrolyte disturbances,
- fever, impaired wound healing.
Very rare:may occur in up to 1 in 10,000 people
- acute toxic dilation of the intestine (toxic megacolon),
- increased pigmentation of the nails, acute paronychia, furunculosis, visible dilation of small blood vessels,
- pain, muscle weakness or numbness, tingling, or decreased reaction to stimuli, changes in taste (metallic taste), seizures, paralysis, meningeal reaction,
- impaired vision, non-inflammatory eye disease (retinopathy),
- decreased libido, impotence, gynecomastia in men, impaired sperm production (oligospermia), menstrual disorders, leukorrhea,
- lymph node enlargement (lymphoma),
- lymphoproliferative disorders (overproduction of white blood cells).
Frequency not known:frequency cannot be estimated from the available data
- increased white blood cell count,
- nosebleeds,
- proteinuria,
- fatigue,
- bone damage in the jaw (as a result of increased white blood cell production),
- destruction of tissue at the injection site,
- redness and peeling of the skin,
- edema.
Subcutaneously administered doses of methotrexate are locally well-tolerated. Only mild local skin reactions have been observed (such as burning sensation, redness, swelling, discoloration, itching, severe itching), which decrease during treatment.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, PL-02-222 Warsaw, phone: +48 22 49-21-301, fax: +48 22 49-21-309,
website: //smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of the medicine.
5. How to store Metex PEN
Keep the medicine out of the sight and reach of children.
Do not store above 25°C. Do not freeze.
Store the pre-filled pen in the outer packaging to protect it from light.
Do not use this medicine after the expiry date stated on the carton and pre-filled pen after "Expiry date". The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Metex PEN contains
- The active substance of the medicine is methotrexate. 1 pre-filled pen with a capacity of 0.15 ml solution contains 7.5 mg of methotrexate. 1 pre-filled pen with a capacity of 0.2 ml solution contains 10 mg of methotrexate. 1 pre-filled pen with a capacity of 0.25 ml solution contains 12.5 mg of methotrexate. 1 pre-filled pen with a capacity of 0.3 ml solution contains 15 mg of methotrexate. 1 pre-filled pen with a capacity of 0.35 ml solution contains 17.5 mg of methotrexate. 1 pre-filled pen with a capacity of 0.4 ml solution contains 20 mg of methotrexate. 1 pre-filled pen with a capacity of 0.45 ml solution contains 22.5 mg of methotrexate. 1 pre-filled pen with a capacity of 0.5 ml solution contains 25 mg of methotrexate.
1 pre-filled pen with a capacity of 0.55 ml solution contains 27.5 mg of methotrexate.
1 pre-filled pen with a capacity of 0.6 ml solution contains 30 mg of methotrexate.
- The other ingredients are sodium chloride, hydrochloric acid, and sodium hydroxide (to adjust pH) and water for injections.
What Metex PEN looks like and contents of the packaging
The medicine is a solution for injection in a pre-filled pen.
The solution is clear, yellow-brown in color.
Metex PEN pre-filled pen is a three-stage automatic pen with a yellow cap and yellow injection button.
Metex PEN pre-filled pen is a two-stage automatic pen with a transparent protective cap and blue needle shield.
The following pack sizes are available:
Metex PEN is available in packs containing 1, 2, 4, 5, 6, 10, 11, 12, 14, 15, or 24 pre-filled pens.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
medac
Gesellschaft für klinische Spezialpräparate mbH
Theaterstr. 6
22880 Wedel
Germany
Phone:
+49 4103 8006-0
Fax:
+49 4103 8006-100
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria, Czech Republic, Finland, Greece, Spain, Netherlands, Slovakia, Slovenia, Hungary, United Kingdom (Northern Ireland):
Metoject PEN
Iceland, Sweden:
Metojectpen
Germany:
metex PEN
Estonia, Lithuania, Latvia, Norway:
Metex
Poland, Portugal:
Metex PEN
Denmark:
Metex Pen
Belgium:
Metoject
Date of last revision of the leaflet: 28-08-2024
Instructions for use
Recommendations
- Before proceeding with the injection, read the following instructions carefully.
- Always use the injection technique recommended by your doctor, pharmacist, or nurse.
Additional information
The method of preparing the medicine for use and disposing of the medicine and the pre-filled pen must comply with local regulations. Pregnant healthcare workers should not prepare or administer the Metex PEN medicinal product.
Methotrexate must not come into contact with skin or mucous membranes. In case of contact, the contaminated area should be rinsed immediately with plenty of water.
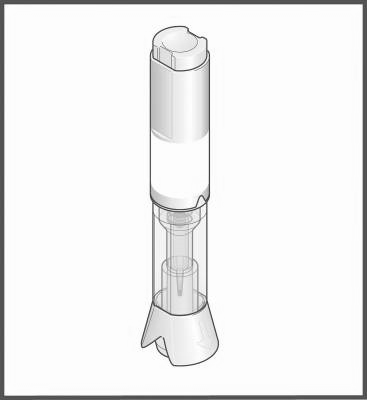
Components of the Metex PEN pen:
Injection button
Area for holding the pen
Transparent control zone
Cap
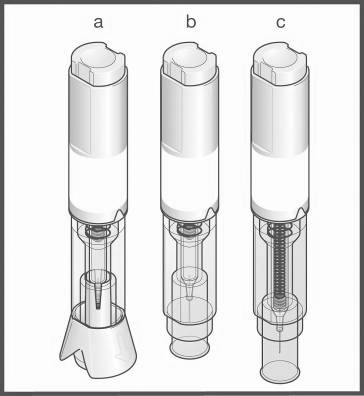
a) With cap before injection
b) After removing cap before injection
c) After injection
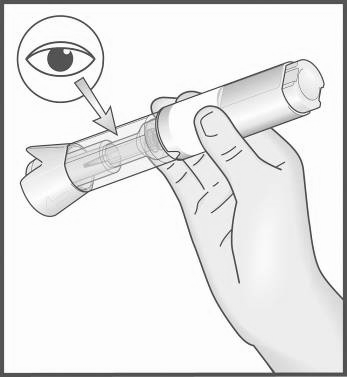
What to do before injecting
- 1. Wash your hands thoroughly.
- 2. Remove the pen from the packaging.
- 3. Check the Metex PEN pen before use:
If the Metex PEN pen appears to be damaged,
do not use it. Use another pen and consult your doctor, pharmacist, or nurse.
If a small air bubble is visible in the transparent control zone, it does not affect the dose or pose a risk to the patient.
If the patient is unable to properly inspect or check the pen before injection, they should ask someone else for help.
- 4. Place the Metex PEN pen on a clean, flat surface (e.g., a table).
Where to inject
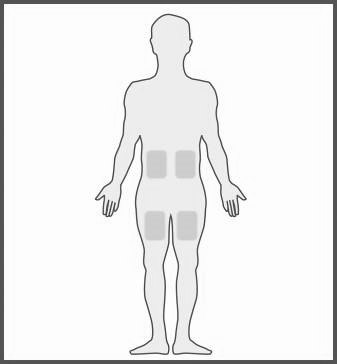
The most suitable injection sites include:
- the upper part of the thigh,
- the abdomen, except for the navel area.
- If someone else is injecting the patient, the injection can also be given in the upper, outer part of the arm, just below the shoulder.
- Each time, choose a different injection site. This will limit the occurrence of a reaction at the injection site.
- Never inject the medicine into a painful, bruised, red, hard, scarred, or stretched area. If the patient has psoriasis, do not inject the medicine directly into raised, thickened, red, or scaly skin lesions or plaques.
How to prepare the injection
- 5. Choose an injection site and clean the selected area and the surrounding skin.
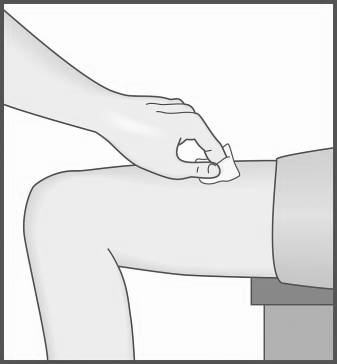
- Do not remove the cap before proceeding with the injection.
- 6. Hold the Metex PEN pen with one hand at the holding area, with the cap facing upwards. With the other hand, gently remove the cap with a straight motion (do not bend or twist the cap). The small needle shield in the cap should automatically slide off with the cap. If the needle shield does not slide off, use another pen and consult your doctor, pharmacist, or nurse.
How to inject:
- 7. Create a skin fold by lightly pinching the cleaned skin area
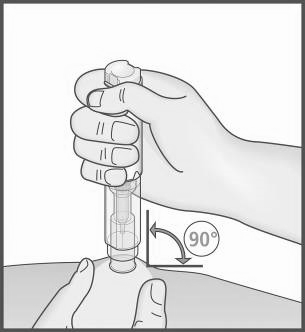
at the injection site.
- Hold the skin fold until the pen is removed from the skin after the injection.
- 8. Place the exposed, transparent end
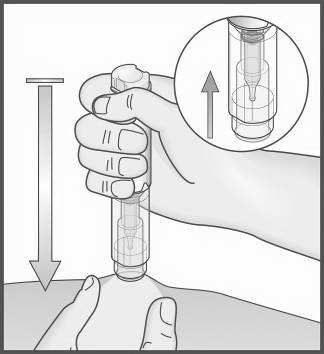
of the Metex PEN pen perpendicularly to the skin fold.
- 9. Without pressing the button, firmly press the Metex PEN pen against the skin to unlock the button.
- If the patient is unable to stabilize the pen at the injection site, they should ask someone else for help.
How to inject:
- 10. While holding the Metex PEN pen firmly against the skin, press the buttonwith your thumb.
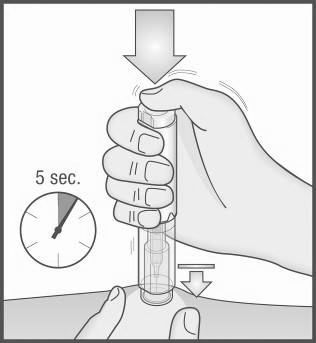
A clicking sound will be heard, indicating the start of the injection. Hold the pen against the skin until the entire dose of medicine has been injected. This may take up to 5 seconds.
Important:
To avoid incomplete injection, do not remove the pen from the skin before the injection is complete.
If the injection is not started, release the button, ensure the pen is firmly pressed against the skin, and press the button firmly.
If you have difficulty hearing the click, count 5 seconds from the button press and then lift the pen from the injection site.
- 11. Remove the Metex PEN pen from the injection site with a perpendicular motion (pull it out).
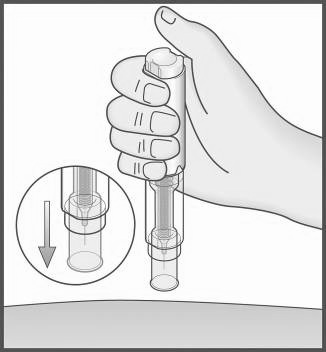
The protective shield will automatically return to cover the needle and will be locked, covering the needle.
- 12. If there is minor bleeding, apply a plaster or bandage.
Before disposing of the Metex PEN pen, visually check if there is any liquid left in the transparentcontrol zone. If there is liquid in the pen, it means that a full dose of the medicine has not been injected, and you should consult your doctor.
Important
To avoid needlestick injuries, never put your fingers into the needle shield opening. Do not destroy the pen.
2. Check the injector before use
(Fig. D)
| Carefully check the name and dose on | |
| the injector and make sure it is the | |
| correct medicine. If the user does not see | |
| well enough, they should ask someone | |
| o pomoc. | |
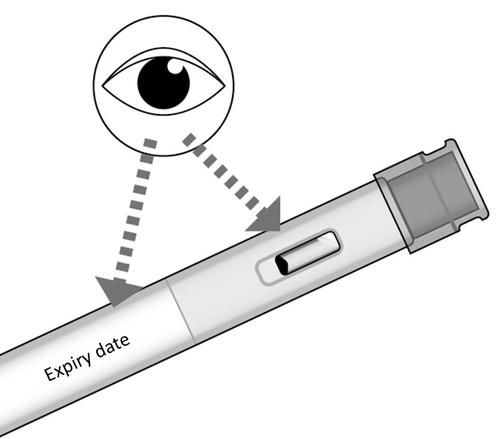
- Check the expiration date on the injector label.
Fig. D
- Do not use the injector if its expiration date has passed.
- Check the medicine through the control window by turning it upside down or gently shaking. The medicine in the injector should be clear and yellow.
- Do not inject if the solution is cloudy, has changed color, or contains solid particles.
- It is normal to see one or a few air bubbles. Do not try to remove them.
- A scale may be visible in the window; do not pay attention to it.
- Check if the injector is damaged and if the cap is properly attached. Do not use the injector if it appears to be damaged or if the cap has been removed or is not properly secured.
If the injector's expiration date has passed, it appears to be damaged, or does not look as expected, you
should not use it and should contact
a medical professional.
Before proceeding with the next steps, carefully place the injector on a clean, flat surface, such as a table.
3. Choose the injection site (Fig. E)
- The patient can perform the injection themselves in:
- the upper part of the thigh,
- the lower part of the abdomen, except for the area within 5 cm of the navel.
- If the injection is performed by a caregiver, they can also use the area on the back of the arm.
- Use a different site than the one used for the last injection.
When choosing the injection site:
do notinject into other parts of the body;
do notinject into bruised, painful, scaly, red, or hardened skin;
do notinject into moles, scars, and stretch marks;
do notinject through clothing.
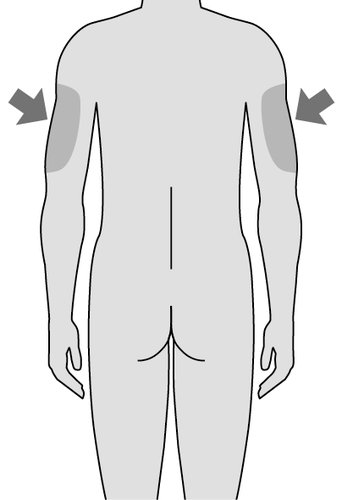
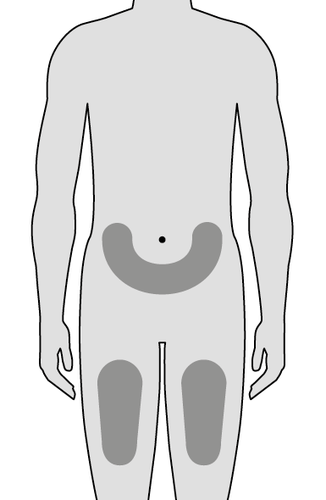
Site
only for
caregiver
Site
only for
caregiver
Fig. E
4. Clean the injection site (Fig. F)
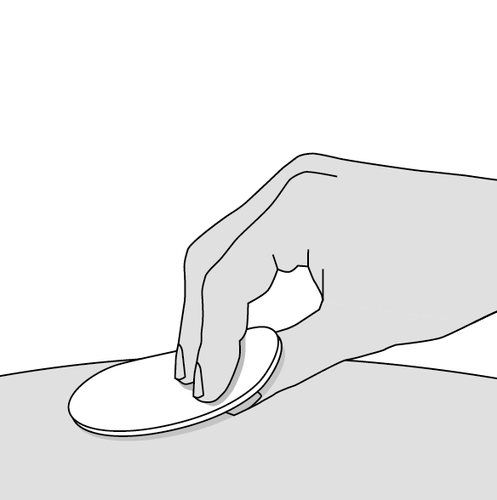
- Clean the injection site with an alcohol-based disinfectant; if not available, you can use water and soap.
- Wait until the skin is dry Do notfan or do notblow on the clean area. Do nottouch the injection site again until the injection is complete.
Fig. F
Injecting the dose
5. Remove the cap (Fig. G)
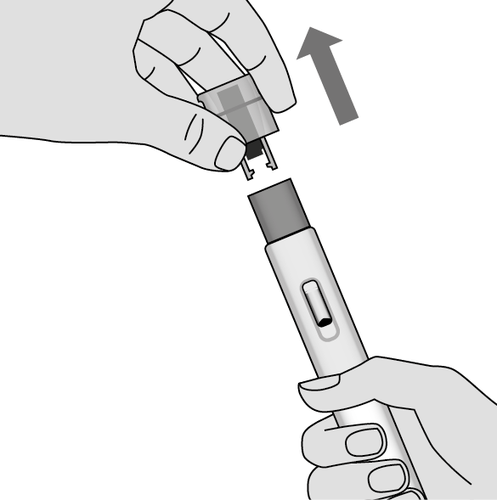
The protective cap should be removed only when the patient is ready to perform the injection.
Do nottry to put the cap back on the injector after removal.
- Hold the injector with the cap facing up and remove the cap with a firm motion.
Fig. G
Do notbend or do nottwist the cap
while removing it.
- Immediately discard the cap.
- Small drops of medicine may appear. This is normal.
- Perform the injection immediately after removing the cap.
Do nottouch the blue needle shield with your fingers.
Touching the blue needle shield can
accidentally trigger the injection
and cause injury.
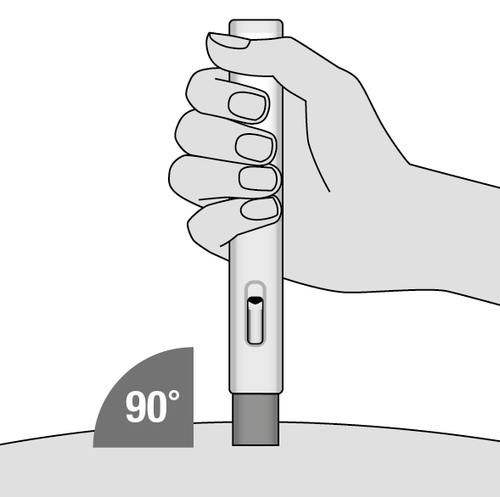
6. Place the injector (Fig. H)
- Place the open blue needle shield on the skin at a 90-degree angle with the control window facing towards you, so it is visible.
- It may be more convenient to grasp the skin fold by gently pinching the area around the injection site between the thumb and the other fingers before performing the injection, but this is not necessary for this injector.
Fig. H
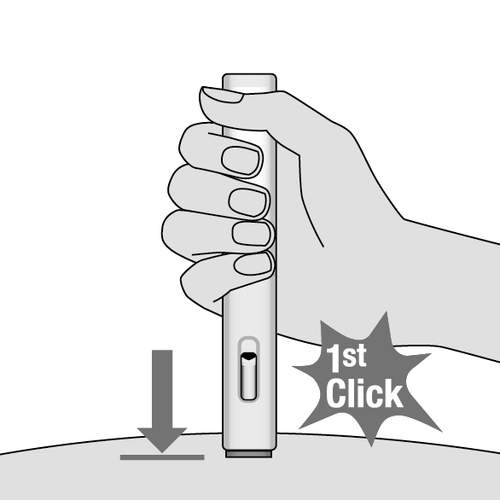
7. Start the injection (Fig. I)
- To start the injection, press the injector fully. This will push the blue needle shield into the injector and the injection will start automatically.
- The first click indicates the start of the injection. The blue plunger body will move down.
Fig. I
- Continue to hold the injector against the skin until the entire dose has been injected. After starting the injection, do notchange the position of the injector.
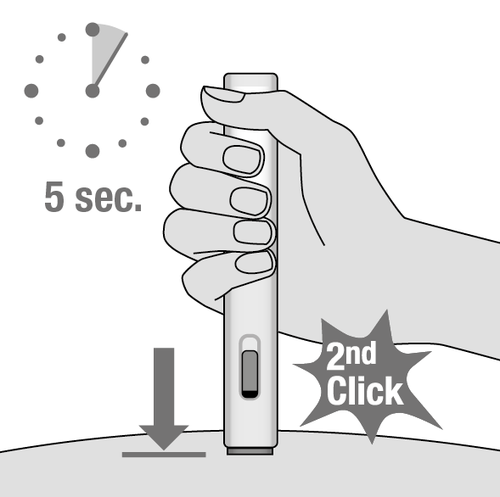
8. Hold the injector in place to
complete the injection (Fig. J)
- Continue to hold the injector against the skin.
5 seconds
- The injection is complete when:
- a second click is heard, shortly after the first;
- or: the blue plunger body has stopped and fills the control window;
- or: 5 seconds have passed.
Fig. J
| Do not remove the injector from the injection site until at least | ||
| 5 seconds have passed. | ||
| 5 sekund. | ||
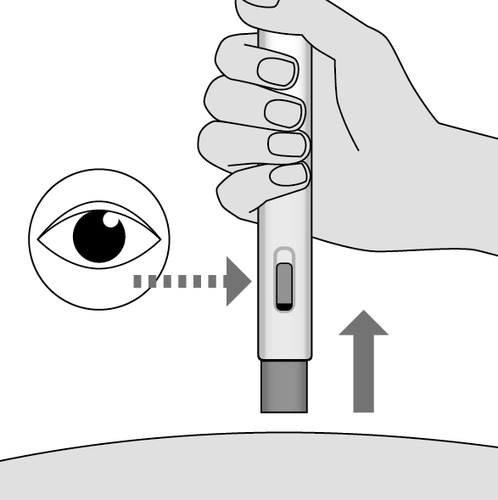
9. Complete the injection (Fig. K)
- Remove the injector straight up from the injection site.
- The blue needle shield will automatically return to its original position around the needle and lock into place.
- Check if there is any remaining yellow medicine in the control window. If you still see yellow medicine in the window, you may not have received the full dose. In this case or if you have any doubts, you should contact a medical professional.
Fig. K
Do nottouch the blue needle shield after
completing the injection, as this may
cause injury.
After the injection
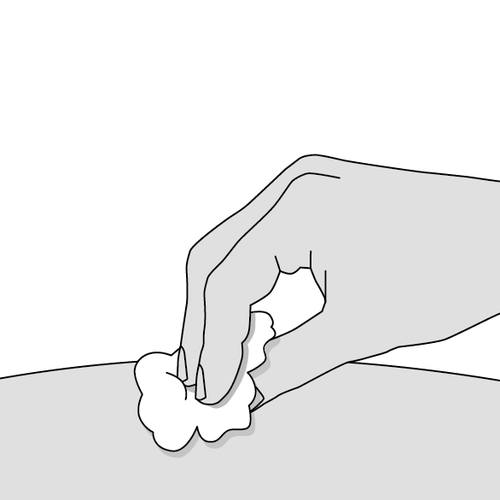
10. Treat the injection site (Fig. L)
- A small drop of blood may appear at the injection site. This is normal. If necessary, press the site with a cotton ball or swab.
- If necessary, cover the injection site with a small bandage.
Do notrub the injection site.
Fig. L
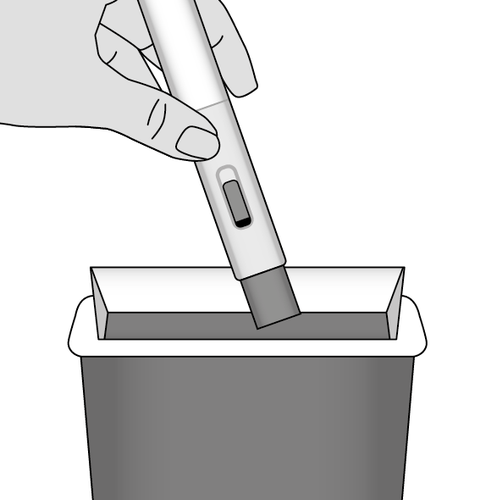
11. Dispose of the injector (Fig. M)
Each injector can only be used once. Do notput the cap back on the injector.
The used injector and protective cap should be stored in a place that is out of sight and inaccessible to children.
Fig. M
- The cap and injector should be disposed of immediately after use. The method of disposing of the medicine and the semi-automatic injector filled must
be in accordance with local regulations.
- Dispose of the used materials in a household waste container. The cardboard box can be thrown away in a paper container.
Metex PEN semi-automatic injectors filled
that have expired, are no longer needed, or are not suitable for use should be disposed of
safely.
- Country of registration
- Active substance
- Prescription requiredYes
- Importermedac Gesellschaft fuer klinische Spezialpraeparate mbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Metex PenDosage form: Solution, 20 mg/mlActive substance: methotrexatePrescription requiredDosage form: Solution, 7.5 mgActive substance: methotrexatePrescription requiredDosage form: Solution, 12.5 mgActive substance: methotrexatePrescription required
Alternatives to Metex Pen in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Metex Pen in Spain
Alternative to Metex Pen in Ukraine
Online doctors for Metex Pen
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Metex Pen – subject to medical assessment and local rules.